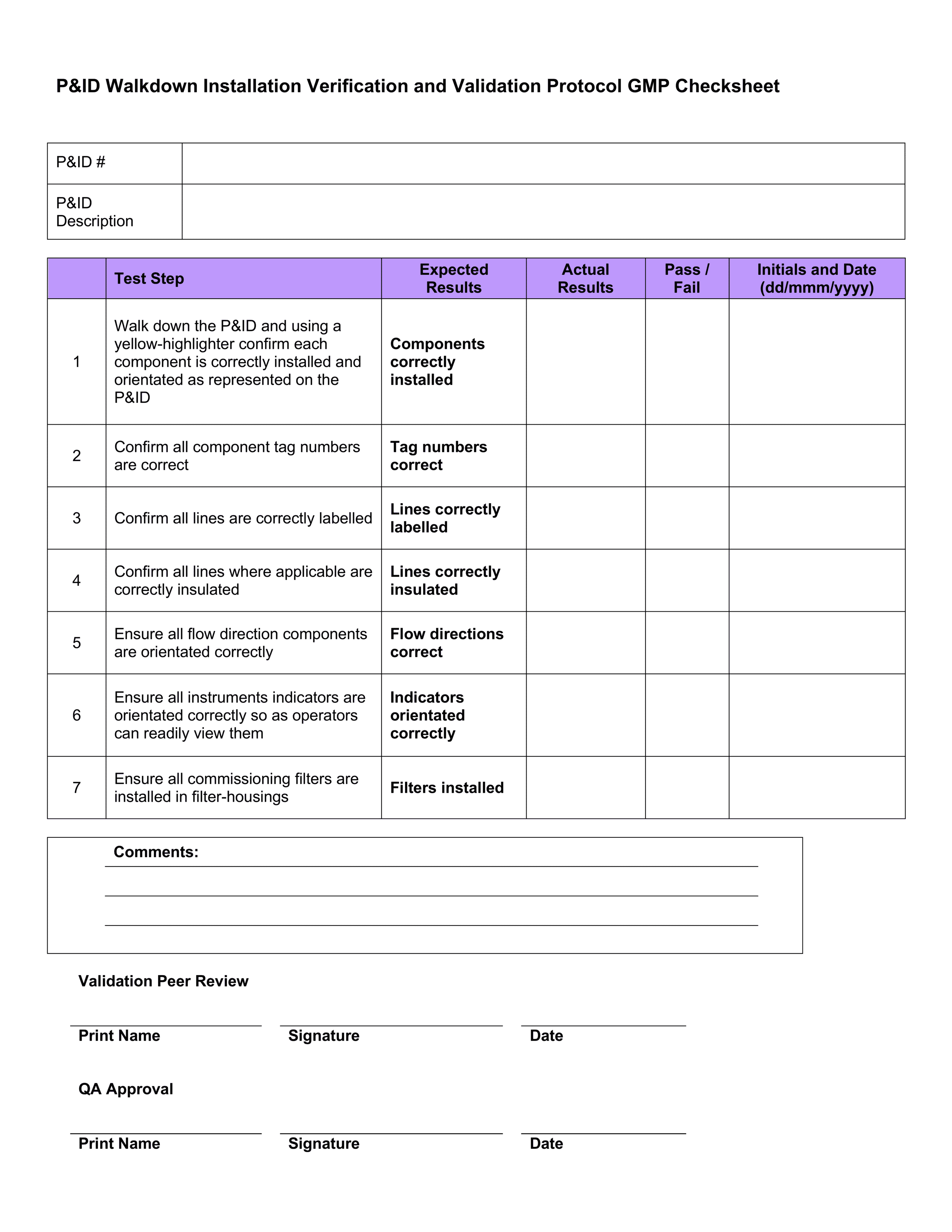
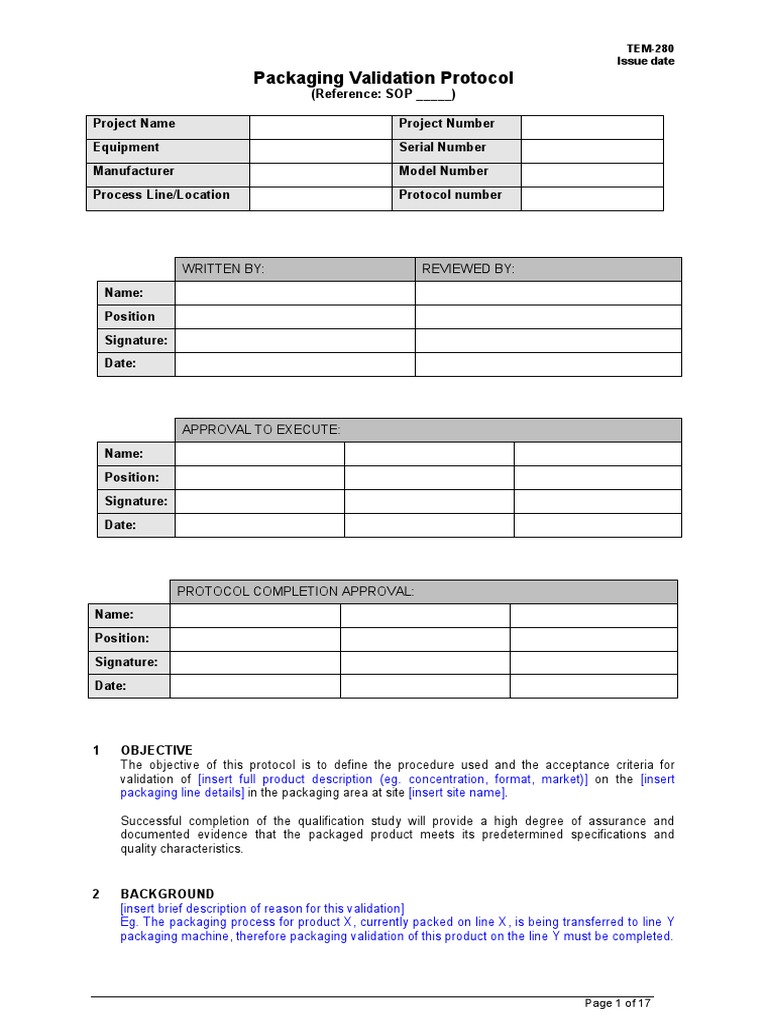
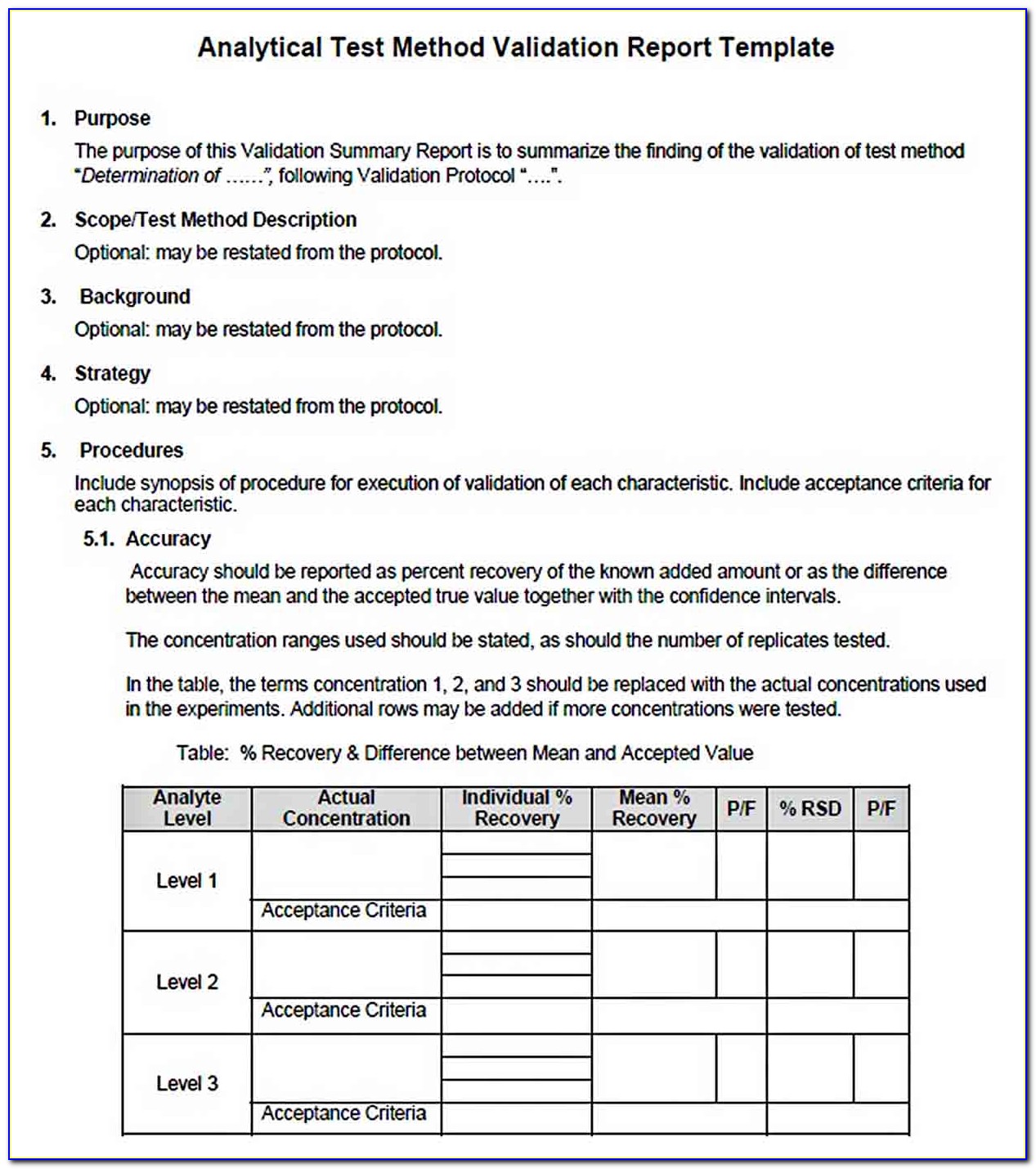
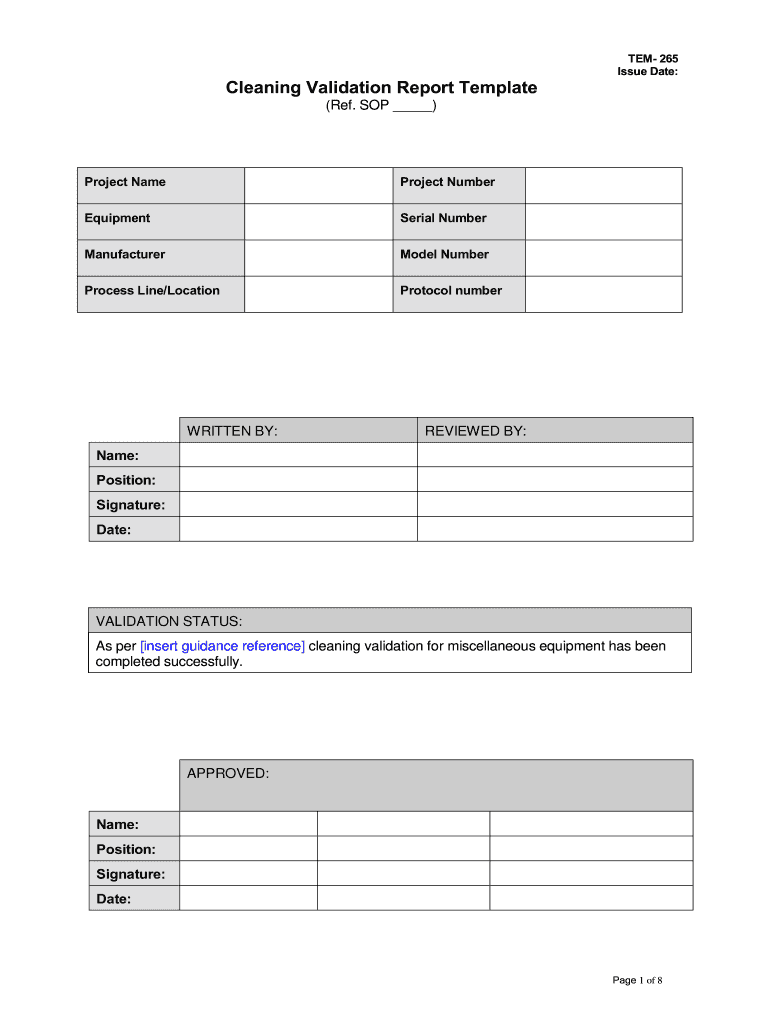
Validation Protocol Template
Validation Protocol Template - That's why having a comprehensive validation protocol sop template is. Web beginner ensuring the accuracy and reliability of your processes is essential for any organization. Web this guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the manufacture of human and animal. Web it is critical to remember that the specifics of the topics covered in this section will be covered in the validation protocols. Web the validation plan and template provided in this document: Purpose of the method, parameters, equipment, procedures, criteria, timeline, and end users. Web this template is used to complete the process validation protocol by reporting the verification of the equipment/system final design against the user,. Web at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is transmitted by the ich assembly to the regulatory. Web this protocol enables you to verify that your developed spreadsheet application is gmp compliant, thus avoiding 483s and warning letters. The validation tasks are explained to the analyst(s) including:
What's a Pharmaceutical Equipment Validation Protocol & Why is it Crucial?
Web this protocol enables you to verify that your developed spreadsheet application is gmp compliant, thus avoiding 483s and warning letters. Web this template is used to complete the process validation protocol by reporting the verification of the equipment/system final design against the user,. Web up to $3 cash back ii. To determine that the equipment/system perform as intended by.
Template of a validation plan. Download Scientific Diagram
Web the validation plan and template provided in this document: Web process validation protocol template or format for the products manufactured in the pharmaceutical product manufacturing facility. Use this equipment validation protocol template to report the. It is a example for the validation protocol. Web this protocol enables you to verify that your developed spreadsheet application is gmp compliant, thus.
TEM280 Packaging Validation Protocol Template Sample Verification
Web this template is used to complete the process validation protocol by reporting the verification of the equipment/system final design against the user,. That's why having a comprehensive validation protocol sop template is. Web this guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the manufacture of human and animal. Web at step.
Excel Spreadsheet Validation Protocol Template —
It also serves as a. Web it is critical to remember that the specifics of the topics covered in this section will be covered in the validation protocols. Use this equipment validation protocol template to report the. • guides the laboratory director in the establishment of method performance specifications considering the intended use. Web fda software validation template software validation.
Sample Cleaning Validation Protocol
You can now validate your application. The method validation plan template is one of the simplest and easiest templates that can help you define the scope and goals of a. Web the validation plan and template provided in this document: Web at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert.
Process Validation Templates at
To determine that the equipment/system perform as intended by repeatedly running the system on its intended. Web up to $3 cash back ii. It also serves as a. Web the validation, verification, and testing plan provides guidance for management and technical efforts throughout the test period. Web validation strategy this process validation will consist of three multi vitamin tablet lots.
Process Validation Protocol for Gliclazide Modified Release Tablets
Web the validation, verification, and testing plan provides guidance for management and technical efforts throughout the test period. Web this guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the manufacture of human and animal. Web fda software validation template software validation for the chemical, manufacturing and cannabis industries what is software validation?.
Template for Process Validation Protocol Verification And Validation
Web this guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the manufacture of human and animal. Use this equipment validation protocol template to report the. Web this protocol enables you to verify that your developed spreadsheet application is gmp compliant, thus avoiding 483s and warning letters. The validation tasks are explained to.
Analytical Method Validation Protocol Sample
That's why having a comprehensive validation protocol sop template is. The method validation plan template is one of the simplest and easiest templates that can help you define the scope and goals of a. Web beginner ensuring the accuracy and reliability of your processes is essential for any organization. It establishes a comprehensive plan to. Web use this process validation.
Cleaning Validation Report Template Fill Online, Printable, Fillable
Web the validation, verification, and testing plan provides guidance for management and technical efforts throughout the test period. Web this guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the manufacture of human and animal. Web the validation plan and template provided in this document: • guides the laboratory director in the establishment.
Web the validation, verification, and testing plan provides guidance for management and technical efforts throughout the test period. Web fda software validation template software validation for the chemical, manufacturing and cannabis industries what is software validation? Web this guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the manufacture of human and animal. Web this template is used to complete the process validation protocol by reporting the verification of the equipment/system final design against the user,. Web use this process validation report template in the pharmaceutical industry to document everything properly. • guides the laboratory director in the establishment of method performance specifications considering the intended use. The method validation plan template is one of the simplest and easiest templates that can help you define the scope and goals of a. Use this equipment validation protocol template to report the. Web this equipment validation protocol template is designed to ensure that the equipment in question is safe, effective, and compliant with applicable regulations. Web at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is transmitted by the ich assembly to the regulatory. Web up to $3 cash back ii. It also serves as a. Web it is critical to remember that the specifics of the topics covered in this section will be covered in the validation protocols. Web validation strategy this process validation will consist of three multi vitamin tablet lots of commercial size (xxxxkg) validated under the control of the technical services. Purpose of the method, parameters, equipment, procedures, criteria, timeline, and end users. It establishes a comprehensive plan to. Validation protocol delete the sections which are not present in xxx system validation protocol, according to validations steps above. The validation tasks are explained to the analyst(s) including: Web the validation plan and template provided in this document: Web process validation protocol template or format for the products manufactured in the pharmaceutical product manufacturing facility.
Purpose Of The Method, Parameters, Equipment, Procedures, Criteria, Timeline, And End Users.
Web this template is used to complete the process validation protocol by reporting the verification of the equipment/system final design against the user,. That's why having a comprehensive validation protocol sop template is. The method validation plan template is one of the simplest and easiest templates that can help you define the scope and goals of a. Web the validation, verification, and testing plan provides guidance for management and technical efforts throughout the test period.
Web Use This Process Validation Report Template In The Pharmaceutical Industry To Document Everything Properly.
Web at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is transmitted by the ich assembly to the regulatory. Web process validation protocol template or format for the products manufactured in the pharmaceutical product manufacturing facility. Web this protocol enables you to verify that your developed spreadsheet application is gmp compliant, thus avoiding 483s and warning letters. You can now validate your application.
Web Fda Software Validation Template Software Validation For The Chemical, Manufacturing And Cannabis Industries What Is Software Validation?
It is a example for the validation protocol. Web this equipment validation protocol template is designed to ensure that the equipment in question is safe, effective, and compliant with applicable regulations. Validation protocol delete the sections which are not present in xxx system validation protocol, according to validations steps above. Use this equipment validation protocol template to report the.
Web This Guidance Outlines The General Principles And Approaches That Fda Considers Appropriate Elements Of Process Validation For The Manufacture Of Human And Animal.
Web up to $3 cash back ii. To determine that the equipment/system perform as intended by repeatedly running the system on its intended. Web beginner ensuring the accuracy and reliability of your processes is essential for any organization. • guides the laboratory director in the establishment of method performance specifications considering the intended use.