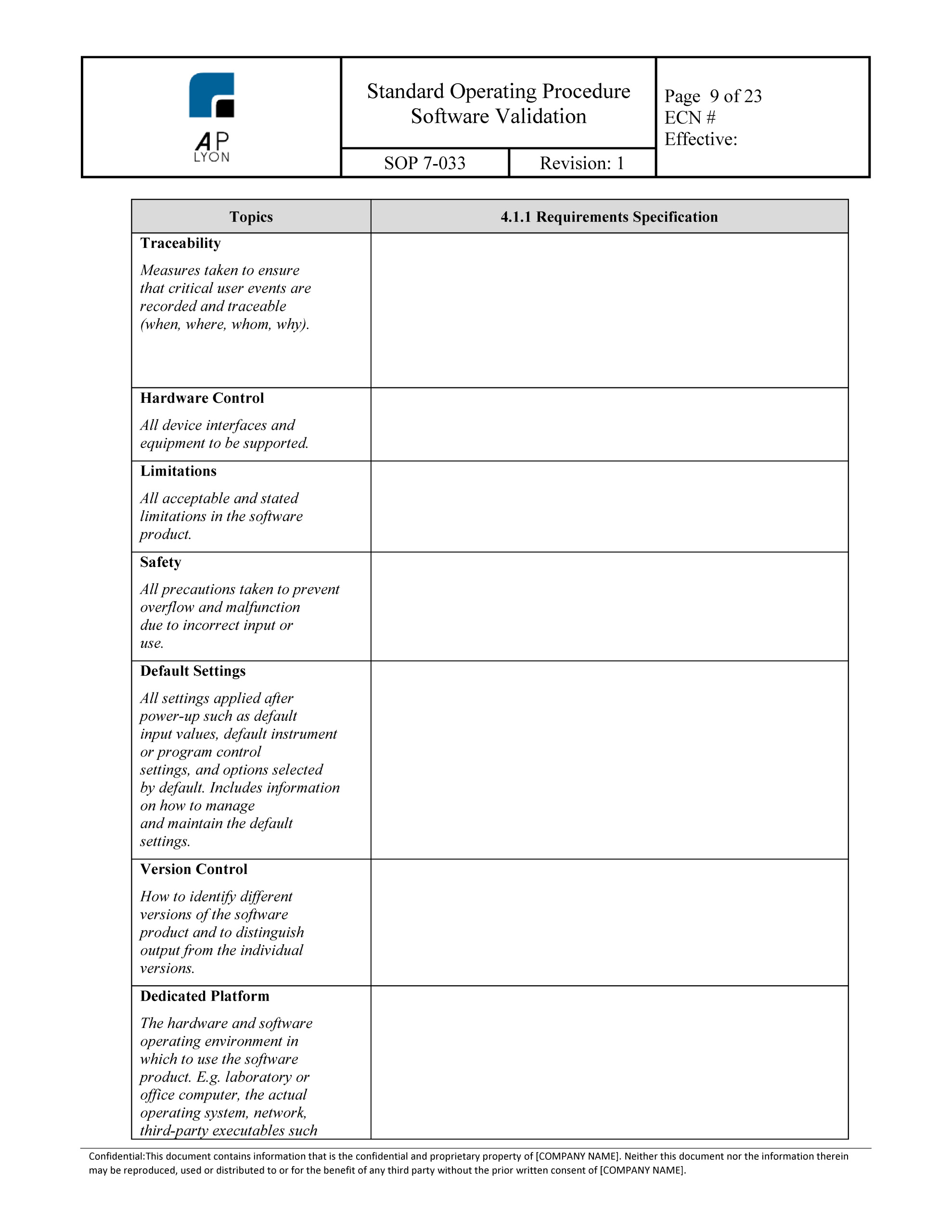
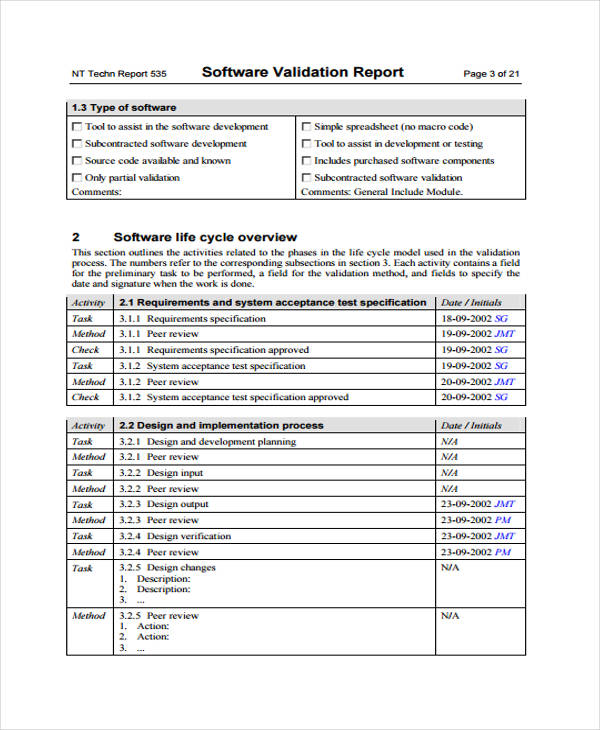
Software Validation Protocol Template
Software Validation Protocol Template - The main messages there are: This template is suitable for authoring the tests of either user. On completion of each validation batch, a qualification report will be prepared. Web standardize validation procedures to maintain consistency and efficiency. System /software requirements specification network. Web software validation protocol (validation plan): Web page 2 guidance for industry and fda staff general principles of software validation in that case, the party with regulatory responsibility (i.e., the device manufacturer) needs to. Web in house written software (excel/access) for eqr, sop and instrument data capture where validation is based on the risk and nature of the software. Basically, software verification activities consist of: Web software validation usually uses three specific testing protocols:
Software Validation Template
General principles of software validation quote: Web software validation protocol (validation plan): A document that outlines the project deliverables and responsibilities. All software changes shall be validatedbefore approval and issuance. Web verification and validation plan template.
Iq Oq Pq Software Validation Templates
Web page 2 guidance for industry and fda staff general principles of software validation in that case, the party with regulatory responsibility (i.e., the device manufacturer) needs to. This template is suitable for authoring the tests of either user. Web operational qualifications (oq) and performance qualifications (pq) test protocols are key validation deliverables. General principles of software validation quote: Use.
SOFTWARE VALIDATION SOP Template PH48 GMP, QSR & ISO Compliance
Web in house written software (excel/access) for eqr, sop and instrument data capture where validation is based on the risk and nature of the software. Web could be safety standard, regulatory standard, customer standards, or company standards. Web standardize validation procedures to maintain consistency and efficiency. Web the complete chain of regulatory required documentation for the software validation template of.
Computer System Validation SOP Validation Center
Web fda software validation template software validation with the chemical, manufacturing and cannabis enterprises what is browse validation? Validate software which is used in. By see tips & download templates, follow us Web the complete chain of regulatory required documentation for the software validation template of a computer system; Web long answer validation of computer software is specified in section.
SOFTWARE VALIDATION SOP Template MD48 GMP, QSR & ISO Compliance
Web learn thing can a software validation according for the fda, 21 cfr part 11 and gamp5. Web operational qualifications (oq) and performance qualifications (pq) test protocols are key validation deliverables. Web same approval signatories as in the validation protocol & validation report. Ad digitize and manage any validation, commissioning or qualification process. Basically, software verification activities consist of:
10+ Validation Report Templates Free Sample, Example Format Download
By see tips & download templates, follow us General principles of software validation quote: Web learn thing can a software validation according for the fda, 21 cfr part 11 and gamp5. The main messages there are: Web standardize validation procedures to maintain consistency and efficiency.
Software Validation Procedure Template
Web standardize validation procedures to maintain consistency and efficiency. Trusted by leading pharma, biotech, and medical device companies globally. Web learn thing can a software validation according for the fda, 21 cfr part 11 and gamp5. Web the complete chain of regulatory required documentation for the software validation template of a computer system; Basically, software verification activities consist of:
Software Validation Templates
On completion of each validation batch, a qualification report will be prepared. Web page 2 guidance for industry and fda staff general principles of software validation in that case, the party with regulatory responsibility (i.e., the device manufacturer) needs to. Web fda software validation template software validation with the chemical, manufacturing and cannabis enterprises what is browse validation? General principles.
Software Validation Protocol Sample
This template is suitable for authoring the tests of either user. The main messages there are: Installation qualifications (iq) verify that systems are on machines suited to run the software, that the system has. Web page 2 guidance for industry and fda staff general principles of software validation in that case, the party with regulatory responsibility (i.e., the device manufacturer).
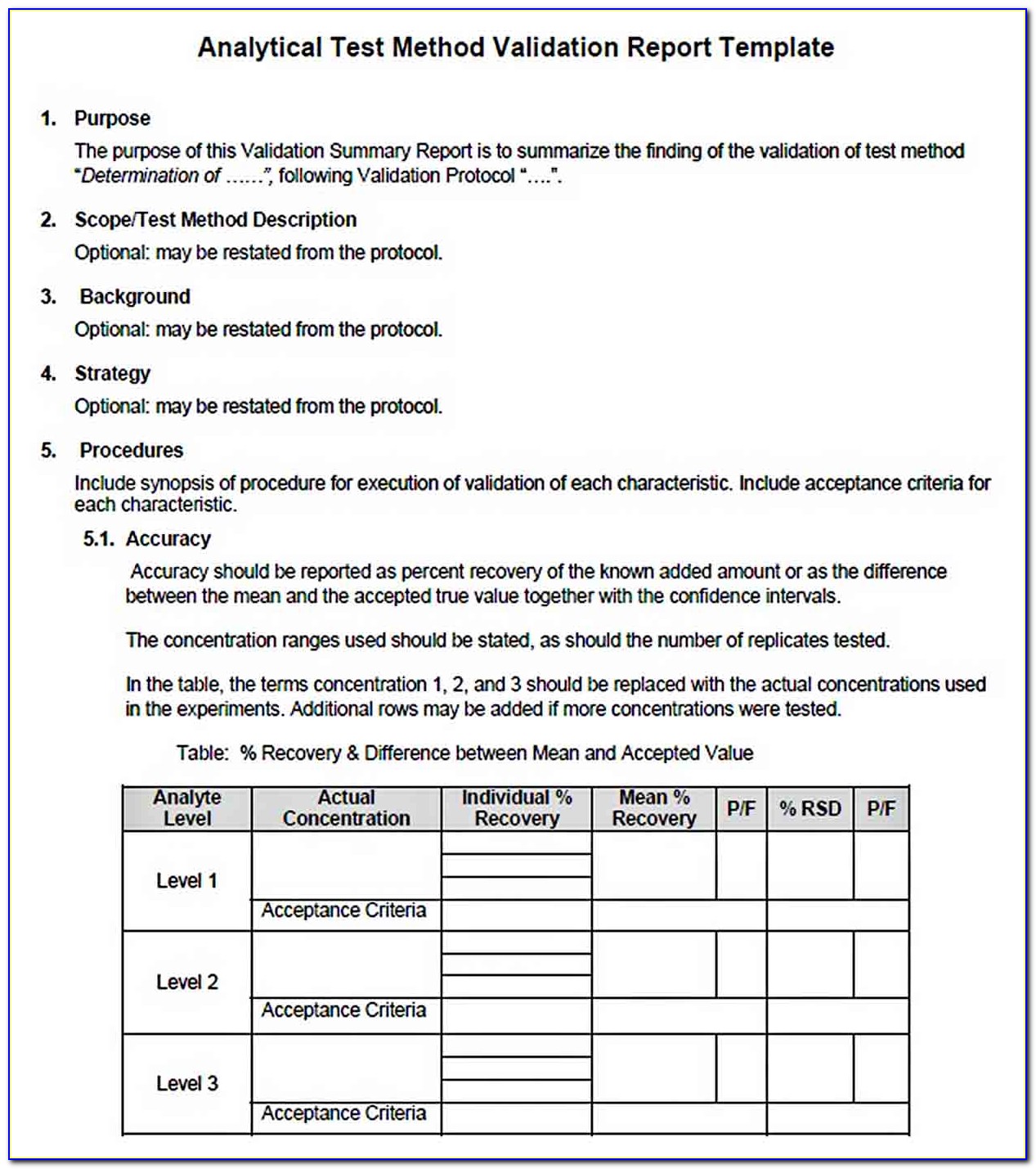
Analytical Method Validation Protocol Sample
Web the complete chain of regulatory required documentation for the software validation template of a computer system; A document that outlines the project deliverables and responsibilities. Web software validation protocol (validation plan): System /software requirements specification network. Web long answer validation of computer software is specified in section 4.1.6 of iso 13485:2016.
Ad digitize and manage any validation, commissioning or qualification process. By see tips & download templates, follow us Web verification and validation plan template. Web standardize validation procedures to maintain consistency and efficiency. Web the complete chain of regulatory required documentation for the software validation template of a computer system; On completion of each validation batch, a qualification report will be prepared. Basically, software verification activities consist of: Web software validation usually uses three specific testing protocols: Web page 2 guidance for industry and fda staff general principles of software validation in that case, the party with regulatory responsibility (i.e., the device manufacturer) needs to. Validate software which is used in. Web could be safety standard, regulatory standard, customer standards, or company standards. Trusted by leading pharma, biotech, and medical device companies globally. Web long answer validation of computer software is specified in section 4.1.6 of iso 13485:2016. Web same approval signatories as in the validation protocol & validation report. Track and document validation activities to meet regulatory requirements. The main messages there are: Web operational qualifications (oq) and performance qualifications (pq) test protocols are key validation deliverables. Web this software verification and validation procedure provides the action steps for the tank waste information network system (twins) testing process. Web fda software validation template software validation with the chemical, manufacturing and cannabis enterprises what is browse validation? Installation qualifications (iq) verify that systems are on machines suited to run the software, that the system has.
Web The Complete Chain Of Regulatory Required Documentation For The Software Validation Template Of A Computer System;
Web long answer validation of computer software is specified in section 4.1.6 of iso 13485:2016. Use this verification and validation plan template to review, inspect, test, audit, and establish whether items, processes, services. On completion of each validation batch, a qualification report will be prepared. Basically, software verification activities consist of:
Trusted By Leading Pharma, Biotech, And Medical Device Companies Globally.
Web this software verification and validation procedure provides the action steps for the tank waste information network system (twins) testing process. All software changes shall be validatedbefore approval and issuance. A document that outlines the project deliverables and responsibilities. General principles of software validation quote:
Web Page 2 Guidance For Industry And Fda Staff General Principles Of Software Validation In That Case, The Party With Regulatory Responsibility (I.e., The Device Manufacturer) Needs To.
Web learn thing can a software validation according for the fda, 21 cfr part 11 and gamp5. Ad digitize and manage any validation, commissioning or qualification process. Web verification and validation plan template. Web in house written software (excel/access) for eqr, sop and instrument data capture where validation is based on the risk and nature of the software.
This Template Is Suitable For Authoring The Tests Of Either User.
Web standardize validation procedures to maintain consistency and efficiency. Web software validation usually uses three specific testing protocols: Track and document validation activities to meet regulatory requirements. Web operational qualifications (oq) and performance qualifications (pq) test protocols are key validation deliverables.