Regulatory Strategy Template For Medical Devices
Regulatory Strategy Template For Medical Devices - Our strategic assessments of regulatory requirements address business needs for medical device, in vitro diagnostic and combination product. I am looking for templates for us, canada and eu regulatory compliance for. Indications for use (ifu) your team should develop an ifu (a basic description of how the device is intended to be used), and should include:. Web here are the guidelines: Web these include your intended use (super important) and your mdr classification document, among others. Web 1 additionally, to successfully navigate the complex regulatory system, recognize and respect factors such as development timelines, budgets, resources and. Ad click now to learn more about rca's global consulting network and service solutions. Contains nonbinding recommendations (version october 6, 2021) 2 Ad management consultants offering the world's best business toolkits, frameworks & templates. Web in this article i'll address design planning, the associated regulatory requirements, and how proper planning should be carried out to control the design of.
Regulatory Strategies for Medical Device Companies to Succeed in Asia
Web #1 does anyone have a regulatory plan template that they would like to share? Web in this article i'll address design planning, the associated regulatory requirements, and how proper planning should be carried out to control the design of. Web feb 18, 2021 strategy for regulatory compliance for mdr with template as european regulatory compliance becomes more complicated many.
Stringent Regulatory Authority The Regulation Of Wearable Medical
This comprises regulatory provisions like registration requirements and processes, but also (not legally binding) guidelines as well as predictive approval. Web feb 18, 2021 strategy for regulatory compliance for mdr with template as european regulatory compliance becomes more complicated many medical device. Web when we are speaking about regulatory strategy for medical devices, three major strategies can cause delays in.
Regulatory Compliance printable pdf download
Web #1 does anyone have a regulatory plan template that they would like to share? Department of health and human services, food and drug. Web background where does regulatory strategy fit in product development? Contains nonbinding recommendations (version october 6, 2021) 2 Establish a robust medical device patient safety net in the united states 2.
Medical Devices; US and Chinese legislation Kvalito
Determining a cost/return on investment for. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them. Ad management consultants offering the world's best business toolkits, frameworks & templates. Web #1 does anyone have a regulatory plan template that they would like to share? Explore regulatory options.
The Medical Device Development Process at DeviceLab Part 1
Department of health and human services, food and drug. Web an effective regulatory compliance strategy for medical devices must contain a number of elements, including: Web #1 does anyone have a regulatory plan template that they would like to share? Web strategy and implementation plan. Web when we are speaking about regulatory strategy for medical devices, three major strategies can.
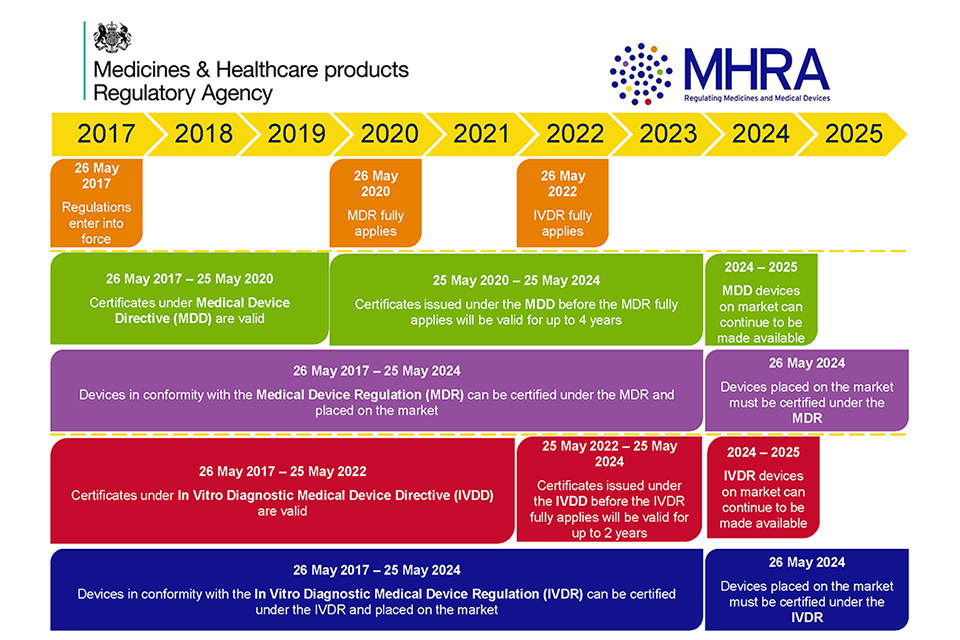
UK MHRA Guidance Medical devices EU regulations for MDR and IVDR
Web strategy and implementation plan. Our strategic assessments of regulatory requirements address business needs for medical device, in vitro diagnostic and combination product. Indications for use (ifu) your team should develop an ifu (a basic description of how the device is intended to be used), and should include:. Web 1 additionally, to successfully navigate the complex regulatory system, recognize and.
Is Regulatory compliance strategy for medical devices effective
Web these include your intended use (super important) and your mdr classification document, among others. Web 1 additionally, to successfully navigate the complex regulatory system, recognize and respect factors such as development timelines, budgets, resources and. Class i, class ii or class iii, based on the level of control necessary to provide reasonable assurance of its safety and. Ad management.
Regulatory Globe Gap Analysis Template Medical Devices, HD Png
Determining a cost/return on investment for. Web in this article i'll address design planning, the associated regulatory requirements, and how proper planning should be carried out to control the design of. Indications for use (ifu) your team should develop an ifu (a basic description of how the device is intended to be used), and should include:. Web these include your.
Image result for design control phases medical device Medical device
Indications for use (ifu) your team should develop an ifu (a basic description of how the device is intended to be used), and should include:. Web 1 additionally, to successfully navigate the complex regulatory system, recognize and respect factors such as development timelines, budgets, resources and. Department of health and human services, food and drug. Our strategic assessments of regulatory.
Fda Design Control Guidance Document
Establish a robust medical device patient safety net in the united states 2. These are the most important ones so you should probably get started. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them. Explore regulatory options to streamline and modernize timely implementation of Class.
Determining a cost/return on investment for. Frameworks, tools & templates to improve your strategic planning capability. Web background where does regulatory strategy fit in product development? The regulatory, quality, compliance and strategy experts your life science you need. Web 1 additionally, to successfully navigate the complex regulatory system, recognize and respect factors such as development timelines, budgets, resources and. Web when we are speaking about regulatory strategy for medical devices, three major strategies can cause delays in obtaining approval to market in any country. Contains nonbinding recommendations (version october 6, 2021) 2 Indications for use (ifu) your team should develop an ifu (a basic description of how the device is intended to be used), and should include:. Ad click now to learn more about rca's global consulting network and service solutions. Web a new requirement for a manufacturer of medical devices and in vitro diagnostics (ivds) is to have a strategy for regulatory compliance. Class i, class ii or class iii, based on the level of control necessary to provide reasonable assurance of its safety and. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them. Department of health and human services, food and drug. Web #1 does anyone have a regulatory plan template that they would like to share? Web here are the guidelines: These are the most important ones so you should probably get started. This comprises regulatory provisions like registration requirements and processes, but also (not legally binding) guidelines as well as predictive approval. Web each device is assigned to one of three regulatory classes: Web feb 18, 2021 strategy for regulatory compliance for mdr with template as european regulatory compliance becomes more complicated many medical device. Establish a robust medical device patient safety net in the united states 2.
Indications For Use (Ifu) Your Team Should Develop An Ifu (A Basic Description Of How The Device Is Intended To Be Used), And Should Include:.
These are the most important ones so you should probably get started. Web when we are speaking about regulatory strategy for medical devices, three major strategies can cause delays in obtaining approval to market in any country. Web a new requirement for a manufacturer of medical devices and in vitro diagnostics (ivds) is to have a strategy for regulatory compliance. This comprises regulatory provisions like registration requirements and processes, but also (not legally binding) guidelines as well as predictive approval.
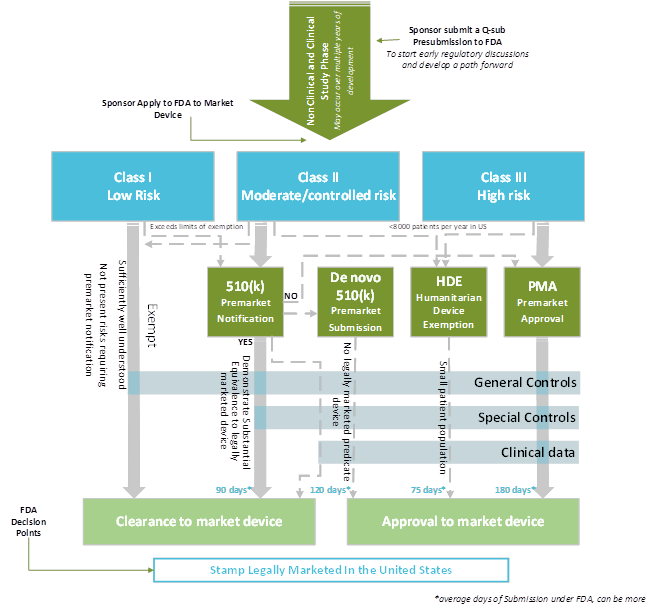
Class I, Class Ii Or Class Iii, Based On The Level Of Control Necessary To Provide Reasonable Assurance Of Its Safety And.
Web please use one of the following formats to cite this article in your essay, paper or report: Web an effective regulatory compliance strategy for medical devices must contain a number of elements, including: Web 1 additionally, to successfully navigate the complex regulatory system, recognize and respect factors such as development timelines, budgets, resources and. Web here are the guidelines:
I Am Looking For Templates For Us, Canada And Eu Regulatory Compliance For.
Web each device is assigned to one of three regulatory classes: Web these include your intended use (super important) and your mdr classification document, among others. Contains nonbinding recommendations (version october 6, 2021) 2 Ad click now to learn more about rca's global consulting network and service solutions.
Establish A Robust Medical Device Patient Safety Net In The United States 2.
Web background where does regulatory strategy fit in product development? Our strategic assessments of regulatory requirements address business needs for medical device, in vitro diagnostic and combination product. Ad management consultants offering the world's best business toolkits, frameworks & templates. The regulatory, quality, compliance and strategy experts your life science you need.