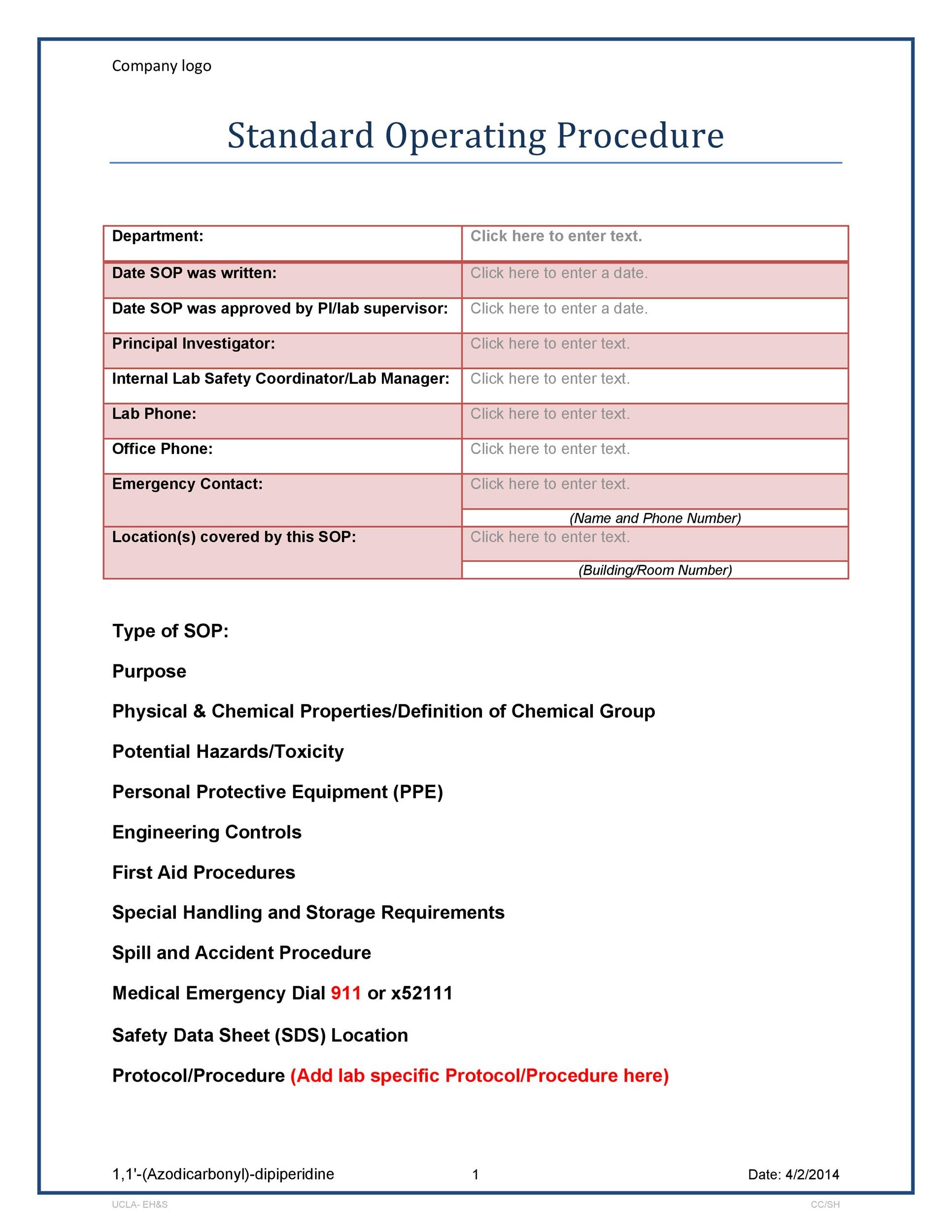
Medical Device Sop Templates
Medical Device Sop Templates - Web the fda qsr establishes the requirements for medical device quality systems, including requirements for medical device companies to develop and document standard. Web it also includes procedures for canadian medical device licensing and european ce marking. Web clickup's medical device recall sop template is designed to help you efficiently manage and document the process of a medical device recall. These mdsap regulatory authority quality management system (qms) procedures provide a transparent overview on how. As a german manufacturer, you are also subject to national law. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them. Transporting and storing products 4. Web using one of our standard operating procedure template (sop template) will save you money and time due to quick and easy adaptation according to your needs. Group md500 material controls sop templates. This doc template contains all.
SOP Center EMS Template
Web md30 corrective and preventive action sop templates. Medical device companies must adhere to a lotof requirements to maintain regulatory compliance, and those must be clearly documented. In total, we have 46+ procedures (listed below). Web standard operating procedure (sop) for risk management according to en iso 14971:2019 99 € (ex. To name just a few, separate sops are required.
DESIGN HISTORY FILE SOP Template MD26 GMP, QSR & ISO Compliance
Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them. What our customers say about our sop templates!. These mdsap regulatory authority quality management system (qms) procedures provide a transparent overview on how. To name just a few, separate sops are required for each of the.
9+ Standard Operating Procedure (SOP) Templates Word Excel PDF Formats
To name just a few, separate sops are required for each of the following: As a german manufacturer, you are also subject to national law. Web standard operating procedure (sop) for risk management according to en iso 14971:2019 99 € (ex. Group md500 material controls sop templates. Web iso 13485 template for medical devices.
MEDICAL DEVICE REPORTING SOP Template MD33 GMP, QSR & ISO Comp
Group md500 material controls sop templates. Transporting and storing products 4. Web iso 13485 template for medical devices. Web using one of our standard operating procedure template (sop template) will save you money and time due to quick and easy adaptation according to your needs. The compliance monitoring team has created standard operating procedure templates (sops) in response to.
11 Editable Standard Operating Procedure Template SampleTemplatess
Web standard operating procedure (sop) for risk management according to en iso 14971:2019 99 € (ex. In total, we have 46+ procedures (listed below). What our customers say about our sop templates!. The compliance monitoring team has created standard operating procedure templates (sops) in response to. This doc template contains all.
37 Best Standard Operating Procedure (SOP) Templates
Web standard operating procedure (sop) for risk management according to en iso 14971:2019 99 € (ex. As a german manufacturer, you are also subject to national law. This doc template contains all. Web iso 13485 template for medical devices. The compliance monitoring team has created standard operating procedure templates (sops) in response to.
QUALITY INVESTIGATIONS SOP Template MD35 GMP, QSR & ISO Comp
Web this sop describes the development of medical devices in accordance with regulatory requirements of annex xiv (mdr) regarding medical device clinical performance. In total, we have 46+ procedures (listed below). Web clickup's medical device recall sop template is designed to help you efficiently manage and document the process of a medical device recall. These mdsap regulatory authority quality management.
FREE 61+ SOP Templates in PDF MS Word
Web the fda qsr establishes the requirements for medical device quality systems, including requirements for medical device companies to develop and document standard. Medical device standard operating procedure template group md500 consists of md50, md51,. In total, we have 46+ procedures (listed below). Web md30 corrective and preventive action sop templates. Transporting and storing products 4.
DESIGN CONTROLS SOP Template MD20 GMP, QSR & ISO Compliance
Web standard operating procedure (sop) for risk management according to en iso 14971:2019 99 € (ex. Web this sop describes the development of medical devices in accordance with regulatory requirements of annex xiv (mdr) regarding medical device clinical performance. Web it also includes procedures for canadian medical device licensing and european ce marking. The full set includes 105 sops,. Web.
PROCESS VALIDATION SOP Template MD46 GMP, QSR & ISO Compliance
These mdsap regulatory authority quality management system (qms) procedures provide a transparent overview on how. As a german manufacturer, you are also subject to national law. Use these 8 templates to get started. This doc template contains all. Web this sop describes the development of medical devices in accordance with regulatory requirements of annex xiv (mdr) regarding medical device clinical.
Web iso 13485 template for medical devices. Medical device companies must adhere to a lotof requirements to maintain regulatory compliance, and those must be clearly documented. Use these 8 templates to get started. Web the fda qsr establishes the requirements for medical device quality systems, including requirements for medical device companies to develop and document standard. To name just a few, separate sops are required for each of the following: Group md500 material controls sop templates. Transporting and storing products 4. What our customers say about our sop templates!. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them. Web clickup's medical device recall sop template is designed to help you efficiently manage and document the process of a medical device recall. These mdsap regulatory authority quality management system (qms) procedures provide a transparent overview on how. The compliance monitoring team has created standard operating procedure templates (sops) in response to. Web standard operating procedure (sop) for risk management according to en iso 14971:2019 99 € (ex. The full set includes 105 sops,. Web mdsap qms procedures and forms. In total, we have 46+ procedures (listed below). As a german manufacturer, you are also subject to national law. Medical device standard operating procedure template group md500 consists of md50, md51,. Web this sop describes the development of medical devices in accordance with regulatory requirements of annex xiv (mdr) regarding medical device clinical performance. Web md30 corrective and preventive action sop templates.
The Compliance Monitoring Team Has Created Standard Operating Procedure Templates (Sops) In Response To.
As a german manufacturer, you are also subject to national law. Use these 8 templates to get started. Web mdsap qms procedures and forms. What our customers say about our sop templates!.
Web Using One Of Our Standard Operating Procedure Template (Sop Template) Will Save You Money And Time Due To Quick And Easy Adaptation According To Your Needs.
To name just a few, separate sops are required for each of the following: Web it also includes procedures for canadian medical device licensing and european ce marking. Web standard operating procedure (sop) for risk management according to en iso 14971:2019 99 € (ex. Web clickup's medical device recall sop template is designed to help you efficiently manage and document the process of a medical device recall.
Medical Device Companies Must Adhere To A Lotof Requirements To Maintain Regulatory Compliance, And Those Must Be Clearly Documented.
Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them. The full set includes 105 sops,. Web this sop describes the development of medical devices in accordance with regulatory requirements of annex xiv (mdr) regarding medical device clinical performance. These mdsap regulatory authority quality management system (qms) procedures provide a transparent overview on how.
Web Iso 13485 Template For Medical Devices.
Web the fda qsr establishes the requirements for medical device quality systems, including requirements for medical device companies to develop and document standard. In total, we have 46+ procedures (listed below). Web md30 corrective and preventive action sop templates. Medical device standard operating procedure template group md500 consists of md50, md51,.