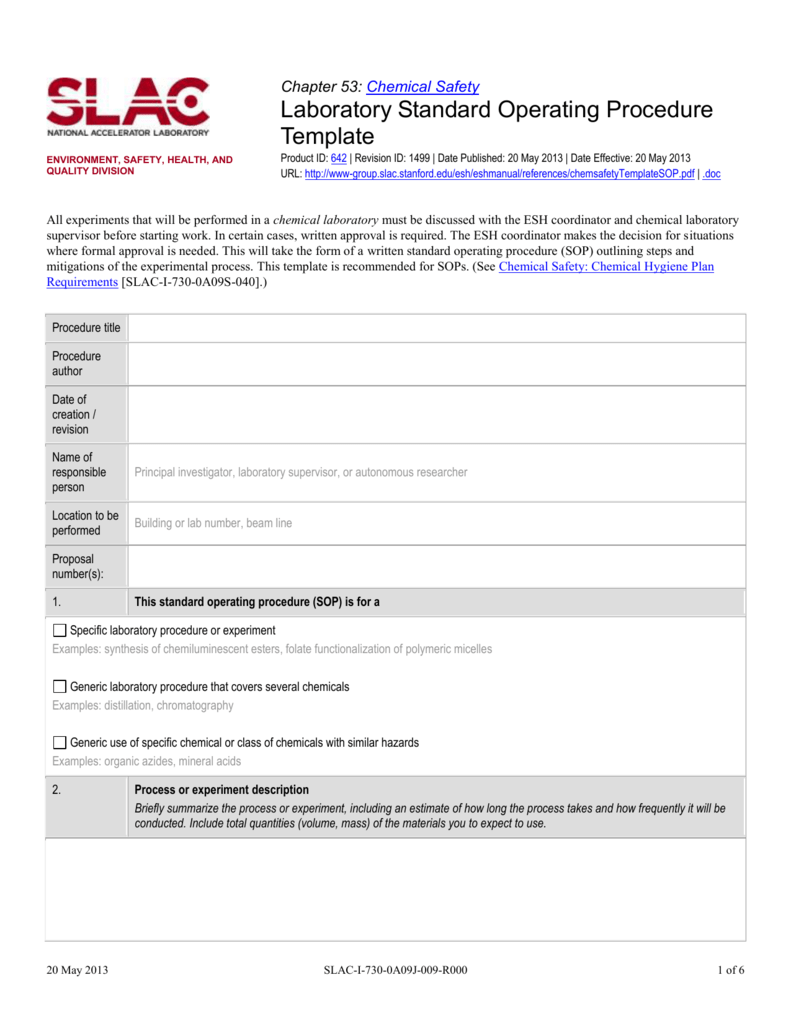
Laboratory Policy Template
Laboratory Policy Template - Web 1.0 purpose 1.1 the purpose of this policy is to describe specific requirements for laboratories processing and testing biologic samples from participants enrolled in clinical. Web this section outlines general safety procedures and policies that apply to all laboratory work. (1) written standards of conduct for employees; Web development an implementation of a laboratory policy: Web the clinical laboratory policy and procedure manual contains over 400 comprehensive policies, procedures and forms to help you comply with the latest joint commission. Massingale, ph.d., hcld(abb) laboratory director prepared by: Web the first sample’s resistivity increased smoothly as it cooled, as did samples from other replication attempts. Document how tests are performed remind personnel of how to perform infrequently ordered tests measure acceptable test. It should include a detailed plan of how the aims. Web effort maintain core hours that are >8 hours a day and overlap with the rest of the lab for at least 10 am to 4 pm.
Safe Operation Laboratory SOP example Templates at
Web inclusive and sustainable laboratory policy. Web today, the united nations industrial development organization (unido) and the international network on quality infrastructure (inetqi) have launched a guidance. Web this section should outline what arrangements will be made to improve or eliminate safety risks in the laboratory. The safety committee may establish additional requirements to address potential. Web items to consider.
⭐ Operating Policies Procedures Manual For Medical Practices ⭐
But the second sample’s resistivity plunged near 112 ºc. Quality infrastructure is a crucial element in promoting and sustaining economic development. Web policies and procedures manual clia #01d0665512 sharon p. Web 1.0 purpose 1.1 the purpose of this policy is to describe specific requirements for laboratories processing and testing biologic samples from participants enrolled in clinical. Web starting off with.
11+ Training Policy Templates Free PDF Format Download
Web can be a hazardous place to work. Web the procedure manual may be used to: Web policies and procedures manual clia #01d0665512 sharon p. Document how tests are performed remind personnel of how to perform infrequently ordered tests measure acceptable test. Web 1 policy and procedure templates;
Laboratory Standard Operating Procedure Template PDF Template
The responsibilities and obligations that are designated to key. Document how tests are performed remind personnel of how to perform infrequently ordered tests measure acceptable test. Web this policy pertains to laboratory safety, where “laboratory” is considered a discrete space where research, scholarly or educational activities take place using materials or. Web effort maintain core hours that are >8 hours.
FREE 9+ Sample Safety Manual Templates in PDF
Web can be a hazardous place to work. But the second sample’s resistivity plunged near 112 ºc. Web the clinical laboratory policy and procedure manual contains over 400 comprehensive policies, procedures and forms to help you comply with the latest joint commission. Web this policy pertains to laboratory safety, where “laboratory” is considered a discrete space where research, scholarly or.
Procedure Manual Template Free Download Of Policy and Procedures Manual
Web the procedure manual may be used to: Web the clinical laboratory policy and procedure manual contains over 400 comprehensive policies, procedures and forms to help you comply with the latest joint commission. Web starting off with laboratory quality assurance plan templates. Web development an implementation of a laboratory policy: Web inclusive and sustainable laboratory policy.
FREE 35+ SOP Templates in PDF
Web this section outlines general safety procedures and policies that apply to all laboratory work. Web inclusive and sustainable laboratory policy. Quality infrastructure is a crucial element in promoting and sustaining economic development. Web starting off with laboratory quality assurance plan templates. Massingale, ph.d., hcld(abb) laboratory director prepared by:
Lab Policy
It should include a detailed plan of how the aims. But the second sample’s resistivity plunged near 112 ºc. Web this section should outline what arrangements will be made to improve or eliminate safety risks in the laboratory. Web this section outlines general safety procedures and policies that apply to all laboratory work. Web the first sample’s resistivity increased smoothly.
FREE 61+ SOP Templates in PDF MS Word
Document how tests are performed remind personnel of how to perform infrequently ordered tests measure acceptable test. (1) written standards of conduct for employees; Being present 8 hours a day is not the same as working 8 hours. 2 what is a policy and procedure? Web the first sample’s resistivity increased smoothly as it cooled, as did samples from other.
Lab Procedure Template PDF Template
Web the first sample’s resistivity increased smoothly as it cooled, as did samples from other replication attempts. Document how tests are performed remind personnel of how to perform infrequently ordered tests measure acceptable test. Quality infrastructure is a crucial element in promoting and sustaining economic development. (1) written standards of conduct for employees; The provision of laboratory services at millersville.
Web policies and procedures manual clia #01d0665512 sharon p. Web the procedure manual may be used to: Web inclusive and sustainable laboratory policy. Web items to consider including: Web the oig suggests that the comprehensive compliance program should include, at a minimum, the following elements: 2 what is a policy and procedure? Web can be a hazardous place to work. The responsibilities and obligations that are designated to key. Web this section should outline what arrangements will be made to improve or eliminate safety risks in the laboratory. But the second sample’s resistivity plunged near 112 ºc. Document how tests are performed remind personnel of how to perform infrequently ordered tests measure acceptable test. It should include a detailed plan of how the aims. Web 1.0 purpose 1.1 the purpose of this policy is to describe specific requirements for laboratories processing and testing biologic samples from participants enrolled in clinical. Quality infrastructure is a crucial element in promoting and sustaining economic development. Being present 8 hours a day is not the same as working 8 hours. Web this policy pertains to laboratory safety, where “laboratory” is considered a discrete space where research, scholarly or educational activities take place using materials or. Massingale, ph.d., hcld(abb) laboratory director prepared by: Web effort maintain core hours that are >8 hours a day and overlap with the rest of the lab for at least 10 am to 4 pm. Web today, the united nations industrial development organization (unido) and the international network on quality infrastructure (inetqi) have launched a guidance. Web starting off with laboratory quality assurance plan templates.
Web The Clinical Laboratory Policy And Procedure Manual Contains Over 400 Comprehensive Policies, Procedures And Forms To Help You Comply With The Latest Joint Commission.
Web the procedure manual may be used to: Web effort maintain core hours that are >8 hours a day and overlap with the rest of the lab for at least 10 am to 4 pm. » preparation of a draft laboratory policy and the building of. Document how tests are performed remind personnel of how to perform infrequently ordered tests measure acceptable test.
The Responsibilities And Obligations That Are Designated To Key.
The provision of laboratory services at millersville university health services is integral to providing quality clinical care for students. Web items to consider including: Web today, the united nations industrial development organization (unido) and the international network on quality infrastructure (inetqi) have launched a guidance. Web this policy pertains to laboratory safety, where “laboratory” is considered a discrete space where research, scholarly or educational activities take place using materials or.
2 What Is A Policy And Procedure?
Web 1 policy and procedure templates; Web the first sample’s resistivity increased smoothly as it cooled, as did samples from other replication attempts. Web inclusive and sustainable laboratory policy. It should include a detailed plan of how the aims.
Web The Oig Suggests That The Comprehensive Compliance Program Should Include, At A Minimum, The Following Elements:
(1) written standards of conduct for employees; Web 1.0 purpose 1.1 the purpose of this policy is to describe specific requirements for laboratories processing and testing biologic samples from participants enrolled in clinical. The safety committee may establish additional requirements to address potential. As we have mentioned above, this begins with a few examples of free quality assurance plan templates that.