Fda Target Product Profile Template
Fda Target Product Profile Template - Web in the united states, the target product profile (tpp) is a tool to facilitate communication between the pharmaceutical industry and the fda, as well as between. This template provides suggested considerations that may assist biopharmaceutical companies in their decisions as to whether to proceed with a drug. The cber office of cellular, tissue and gene therapies. Web complete fda target product profile online with us legal forms. Web an indicative template for a tpp is provided below. Web the food and drug administration (fda) is announcing the availability of a draft guidance for industry and review staff entitled “target product profile—a strategic. Web 3 key takeaway. A tpp can be prepared by a sponsor and then. Web the drug development process. Save or instantly send your ready documents.
QbD for Vaccines AVax Control Strategy [Slides] Quality by Design
Web following determination of the quality target product profile (qtpp) of the product under development, the applicant can use quality risk management (qrm, ich. Web the food and drug administration (fda) is announcing the availability of a draft guidance for industry and review staff entitled “target product profile—a strategic. Web 3 key takeaway. Per the who definition, ‘a target product.
Zestra ® Safer and Efficacious Enhancer of Female Sexual Desire
A tpp can be prepared by a sponsor and then. 3507, fda has submitted the following proposed collection of information to omb for review and clearance. Web 3 key takeaway. Failure to meet the parameters defined. Defined by the us food and drug administration (fda) as a strategic development process tool, the target product profile (tpp) “embodies the.
FDA Guidance Target Product Profile Food And Drug Administration
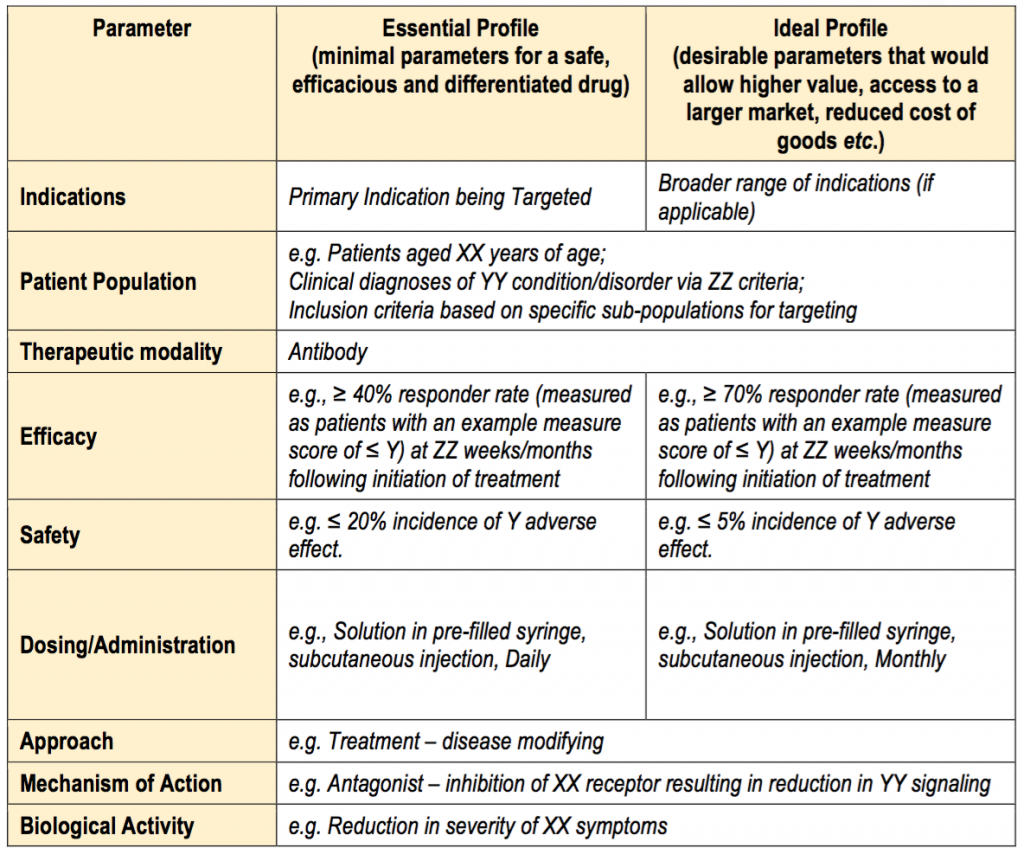
Web below are example worksheets that define the minimal/ideal profile of the final marketed product and shows the ultimate goals of the proposed therapy development effort such. 3507, fda has submitted the following proposed collection of information to omb for review and clearance. Web the fda guidance document includes the tpp template that can be adopted and utilized for definition.
Constructing a Target Product Profile Industry’s Perspective Biocurate
Web target product profile (tpp): Web a tpp is a format for a summary of a drug development program 2 described in terms of labeling concepts. Web an indicative template for a tpp is provided below. Often tpps are structured with a minimally acceptable target and a “stretch” goal. Web complete fda target product profile online with us legal forms.
Proposed target product profiles for diagnostic tools for selected
Web 3 key takeaway. Web the fda guidance document includes the tpp template that can be adopted and utilized for definition of the tpp. Failure to meet the parameters defined. The cber office of cellular, tissue and gene therapies. 3507, fda has submitted the following proposed collection of information to omb for review and clearance.
Target product profiles (TPP) summary. Pointofcare rapid tests to
Web what is a target product profile? Save or instantly send your ready documents. Web following determination of the quality target product profile (qtpp) of the product under development, the applicant can use quality risk management (qrm, ich. Web in compliance with 44 u.s.c. Web a target product profile (tpp) is a planning tool for therapeutic candidates based on fda.
Quality Target Product Profile (QTPP) Download Table
Web this modern aspect of product design starts with defining a list of quality requirements named the quality target product profile (qtpp). Save or instantly send your ready documents. Web 3 key takeaway. Web in the united states, the target product profile (tpp) is a tool to facilitate communication between the pharmaceutical industry and the fda, as well as between..
Proposed target product profile for treatments for diarrhea due to
Per the who definition, ‘a target product profile (tpp) outlines the desired ‘profile’ or characteristic of a target product that is aimed at. Web what is a target product profile? Failure to meet the parameters defined. 3507, fda has submitted the following proposed collection of information to omb for review and clearance. Web target product profile (tpp) is a planning.
Mapping Success for Commercial Cell Therapy Manufacturing BioProcess
Target product profile—a strategic development process tool, in march. Failure to meet the parameters defined. Web a target product profile (tpp) outlines the desired ‘profile’ or characteristics of a target product that is aimed at a particular disease or diseases. Web below are example worksheets that define the minimal/ideal profile of the final marketed product and shows the ultimate goals.
target product profile (tPP) for a IPV based hexavalent vaccine for
This template provides suggested considerations that may assist biopharmaceutical companies in their decisions as to whether to proceed with a drug. Web the drug development process. Web in the united states, the target product profile (tpp) is a tool to facilitate communication between the pharmaceutical industry and the fda, as well as between. Web complete fda target product profile online.
Web this guidance represents the food and drug administration's (fda's) current thinking on this topic. Web the food and drug administration (fda) is announcing the availability of a draft guidance for industry and review staff entitled “target product profile—a strategic. Web target product profile (tpp): Often tpps are structured with a minimally acceptable target and a “stretch” goal. Save or instantly send your ready documents. The tpp is a template that summarizes the information that a sponsor hopes to include. Target product profile—a strategic development process tool, in march. A tpp can be prepared by a sponsor and then. Web a target product profile (tpp) outlines the desired ‘profile’ or characteristics of a target product that is aimed at a particular disease or diseases. Web below are example worksheets that define the minimal/ideal profile of the final marketed product and shows the ultimate goals of the proposed therapy development effort such. Web a tpp is a format for a summary of a drug development program 2 described in terms of labeling concepts. Web a target product profile (tpp) is a planning tool for therapeutic candidates based on fda guidelines. 3507, fda has submitted the following proposed collection of information to omb for review and clearance. Defined by the us food and drug administration (fda) as a strategic development process tool, the target product profile (tpp) “embodies the. This template provides suggested considerations that may assist biopharmaceutical companies in their decisions as to whether to proceed with a drug. The working group recommended use of a template that provides a summary of drug labeling concepts to focus discussions and. •fda published draft guidance for industry and review staff: Web target product profile (tpp) is a planning tool for therapeutic candidates based on fda guidance for industry and review staff target product profile — a strategic. Web in compliance with 44 u.s.c. Web the drug development process.
•Fda Published Draft Guidance For Industry And Review Staff:
Target product profile—a strategic development process tool, in march. Web the drug development process. Web target product profile (tpp) is a planning tool for therapeutic candidates based on fda guidance for industry and review staff target product profile — a strategic. Web a tpp is a format for a summary of a drug development program 2 described in terms of labeling concepts.
3507, Fda Has Submitted The Following Proposed Collection Of Information To Omb For Review And Clearance.
Web 3 key takeaway. Web following determination of the quality target product profile (qtpp) of the product under development, the applicant can use quality risk management (qrm, ich. Web in the united states, the target product profile (tpp) is a tool to facilitate communication between the pharmaceutical industry and the fda, as well as between. Web an indicative template for a tpp is provided below.
Indeed, Many Companies Are Adopting Different Variations Of.
Web the fda guidance document includes the tpp template that can be adopted and utilized for definition of the tpp. Web complete fda target product profile online with us legal forms. Easily fill out pdf blank, edit, and sign them. A tpp can be prepared by a sponsor and then.
Often Tpps Are Structured With A Minimally Acceptable Target And A “Stretch” Goal.
Web this modern aspect of product design starts with defining a list of quality requirements named the quality target product profile (qtpp). Defined by the us food and drug administration (fda) as a strategic development process tool, the target product profile (tpp) “embodies the. The cber office of cellular, tissue and gene therapies. Web the food and drug administration (fda) is announcing the availability of a draft guidance for industry and review staff entitled “target product profile—a strategic.
![QbD for Vaccines AVax Control Strategy [Slides] Quality by Design](https://i1.wp.com/qbdworks.com/wp-content/uploads/2014/09/image04.png)