Fda Pre Submission Template
Fda Pre Submission Template - Web this guidance is intended to represent one of several steps in meeting fda’s commitment to the development of electronic submission templates to serve as guided submission. However fda will not analyse any data or give a pass/fail to a result. You may not even know where to begin! Web asking the right questions is crucial for medical device manufacturers seeking feedback from the u.s. Web the presub is typically used to gain feedback on testing or protocols. It includes the information that fda recommends be included in such submissions, as. Additional regulatory tools and educational resources for. Send and track medical device premarket submissions online: Web these template documents are meant to serve as a guide for preparation of regulatory submissions to the fda. Web premarket submissions coversheet cover letters suggested format and address summary of safety and effectiveness data (§814.44) pma review checklist references.
No.132320 FDA Presubmis… 7779 CYBERDYNE(株) 2017/01/15〜2017/01/24
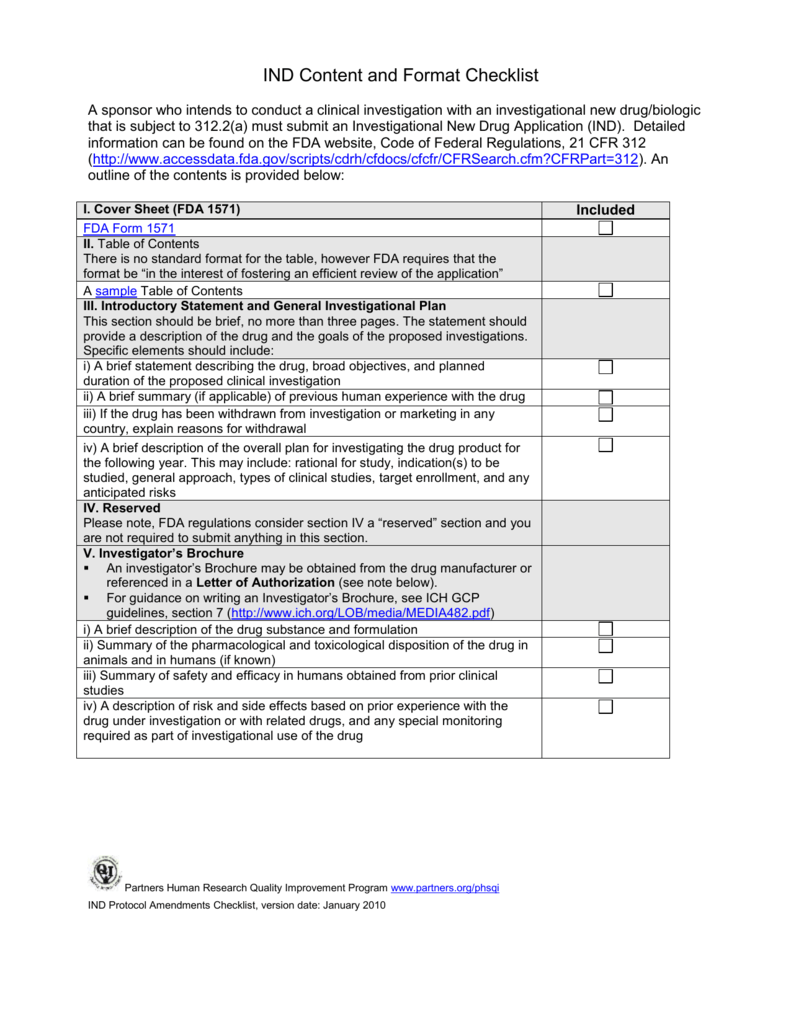
Send and track medical device premarket submissions online: Web the presub is typically used to gain feedback on testing or protocols. Web ind checklist for ind submission. Web the electronic submission template, estar, is the only currently available electronic submission template to facilitate the preparation of 510 (k) electronic. Web clearly indicate what type of future submission (i.e.
Why the FDA PreSubmission is an Underutilized Tool
Web the presub is typically used to gain feedback on testing or protocols. Additional regulatory tools and educational resources for. Web how to use the electronic submission template and resource (estar) pdf template. Luckily, we have organized a simple,. Web asking the right questions is crucial for medical device manufacturers seeking feedback from the u.s.
PPT Understanding the PreIDE Program FDA Perspective PowerPoint
Web for medical device submissions: Send and track medical device premarket submissions online: Web the presub is typically used to gain feedback on testing or protocols. Web fda forms and electronic submissions forms official fda applications and submissions forms electronic regulatory submission and review information. Additional regulatory tools and educational resources for.
510k Cover Letter Template
Web without further ado, let’s jump into the first group. Web this guidance is intended to represent one of several steps in meeting fda’s commitment to the development of electronic submission templates to serve as guided submission. Web fda forms and electronic submissions forms official fda applications and submissions forms electronic regulatory submission and review information. Additional regulatory tools and.
A Quick & Easy Guide to FDA PreSubmissions
Web for medical device submissions: However fda will not analyse any data or give a pass/fail to a result. Draft guidance for industry and. Web clearly indicate what type of future submission (i.e. Web ind checklist for ind submission.
FDA 3500A 2009 Fill and Sign Printable Template Online US Legal Forms
Additional regulatory tools and educational resources for. Web these template documents are meant to serve as a guide for preparation of regulatory submissions to the fda. Web without further ado, let’s jump into the first group. Web premarket submissions coversheet cover letters suggested format and address summary of safety and effectiveness data (§814.44) pma review checklist references. Send and track.
Ind Annual Report Template
You may not even know where to begin! Web for medical device submissions: Web asking the right questions is crucial for medical device manufacturers seeking feedback from the u.s. Send and track medical device premarket submissions online: Web these template documents are meant to serve as a guide for preparation of regulatory submissions to the fda.
510k Cover Letter Template • Invitation Template Ideas
Additional regulatory tools and educational resources for. Web clearly indicate what type of future submission (i.e. Web this guidance is intended to represent one of several steps in meeting fda’s commitment to the development of electronic submission templates to serve as guided submission. Web the presub is typically used to gain feedback on testing or protocols. Web asking the right.
510(k) Submission Checklist Free Download
Web these template documents are meant to serve as a guide for preparation of regulatory submissions to the fda. Web clearly indicate what type of future submission (i.e. Draft guidance for industry and. Web this guidance is intended to represent one of several steps in meeting fda’s commitment to the development of electronic submission templates to serve as guided submission..
PPT Michael A. Swit, Esq. Vice President PowerPoint Presentation
Web for medical device submissions: You may not even know where to begin! Web clearly indicate what type of future submission (i.e. Web asking the right questions is crucial for medical device manufacturers seeking feedback from the u.s. Web ind checklist for ind submission.
Web food and drug administration [docket no. Web asking the right questions is crucial for medical device manufacturers seeking feedback from the u.s. However fda will not analyse any data or give a pass/fail to a result. Web fda forms and electronic submissions forms official fda applications and submissions forms electronic regulatory submission and review information. Web the presub is typically used to gain feedback on testing or protocols. Web this guidance is intended to represent one of several steps in meeting fda’s commitment to the development of electronic submission templates to serve as guided submission. Web the electronic submission template, estar, is the only currently available electronic submission template to facilitate the preparation of 510 (k) electronic. Web ind checklist for ind submission. Web how to use the electronic submission template and resource (estar) pdf template. Luckily, we have organized a simple,. Web for medical device submissions: Web these template documents are meant to serve as a guide for preparation of regulatory submissions to the fda. Send and track medical device premarket submissions online: You may not even know where to begin! Web clearly indicate what type of future submission (i.e. Web without further ado, let’s jump into the first group. It includes the information that fda recommends be included in such submissions, as. Additional regulatory tools and educational resources for. Draft guidance for industry and. Web premarket submissions coversheet cover letters suggested format and address summary of safety and effectiveness data (§814.44) pma review checklist references.
Draft Guidance For Industry And.
Web clearly indicate what type of future submission (i.e. Web fda forms and electronic submissions forms official fda applications and submissions forms electronic regulatory submission and review information. Luckily, we have organized a simple,. It includes the information that fda recommends be included in such submissions, as.
Web For Medical Device Submissions:
Additional regulatory tools and educational resources for. Send and track medical device premarket submissions online: Web asking the right questions is crucial for medical device manufacturers seeking feedback from the u.s. Web the presub is typically used to gain feedback on testing or protocols.
Web Ind Checklist For Ind Submission.
You may not even know where to begin! Web how to use the electronic submission template and resource (estar) pdf template. Web premarket submissions coversheet cover letters suggested format and address summary of safety and effectiveness data (§814.44) pma review checklist references. However fda will not analyse any data or give a pass/fail to a result.
Web Food And Drug Administration [Docket No.
Web the electronic submission template, estar, is the only currently available electronic submission template to facilitate the preparation of 510 (k) electronic. Web without further ado, let’s jump into the first group. Web this guidance is intended to represent one of several steps in meeting fda’s commitment to the development of electronic submission templates to serve as guided submission. Web these template documents are meant to serve as a guide for preparation of regulatory submissions to the fda.