Device Master Record Template
Device Master Record Template - Web the device master record (dmr) is focused on building the device and ensuring that all necessary items are included to build, test, package, and service it. Web adenine device master register (dmr) contain all the information required to builds your appliance from start to finish. Web a device master record (dmr) does all the information requires to build your device out start to finish. Learn what default is integrated along with specifications, drawing,. Web device master record (dmr) is the term used in the quality system (qs) regulation for all of the routine documentation required to manufacture devices that will consistently meet. Upon completion of the design phase of a device, a formal. Web against compilation of all related to be included in the master record, a device master record index should breathe prepared to identify all items press locations. The term is used in quality management systems that cover product design and production. Web a device master record (dmr) contains all the information required to build your device from start to close. Related to the device itself:
Device Master Records.doc Specification (Technical Standard
This white paper focuses on medical device compliance per 21 cfr 820.181 for dmr and iso 13485:2016 § 4.2.3. Web a device master record (dmr) is a collection of records that contains the procedures and specifications for a finished medical device. Each manufacturer shall ensure that each dmr is prepared and. Let’s imagine that your medical devices. Web a device.
8 DEVICE MASTER RECORDS
Details on formulation & composition. Learn which else is included along with specifications, drawing, the. Let’s imagine that your medical devices. A device master record (dmr) is a compilation of all the instructions, drawings and other records that must be used to produce a product. Learn what else a included along with specifications,.
Device Master Record Contents Template
Web against compilation of all related to be included in the master record, a device master record index should breathe prepared to identify all items press locations. Identify key definitions related to documents and records 2. Upon completion of the design phase of a device, a formal. A device master record (dmr) is a compilation of all the instructions, drawings.
DEVICE MASTER RECORD SOP Template MD21 GMP, QSR & ISO Comp
Let’s imagine that your medical devices. Web a device master record (dmr) contains all the information required to build your device from start to close. Each manufacturer shall maintain device master records (dmr's). Web adenine device master register (dmr) contain all the information required to builds your appliance from start to finish. Web the device master record contents template is.
Medical Device Master File Template
Web device master record (dmr) is the term used in the quality system (qs) regulation for all of the routine documentation required to manufacture devices that will consistently meet. Web the device master record (dmr) is focused on building the device and ensuring that all necessary items are included to build, test, package, and service it. Each manufacturer shall ensure.
Device Master Record Procedure
Web the device master record contents template is a listing of items that may appear in a device master record. Web a device master record (dmr) contains all the information required to build your device from start to close. Web a device master record (dmr) is a collection of records that contains the procedures and specifications for a finished medical.
Medical Device Master File Template alat press tutup gelas plastik murah
The term is used in quality management systems that cover product design and production. Web adenine device master register (dmr) contain all the information required to builds your appliance from start to finish. Web against compilation of all related to be included in the master record, a device master record index should breathe prepared to identify all items press locations..
Device Master Records & Design History Files
Learn what default is integrated along with specifications, drawing,. Web the device master record contents template is a listing of items that may appear in a device master record. There is quite a big overlap between the two documents, but basically we can say that the medical device file requested by the iso 13485 corresponds to the dmr (a typical.
Device Master Record
Web section 820.3(j) of the federal code defines device master record. Learn what default is integrated along with specifications, drawing,. Web each manufacturer shall maintain device master records (dmr's). Describe requirements and intent for document controls,. Web a device master record (dmr) is a collection of all the records that must be used to produce a medical device product.
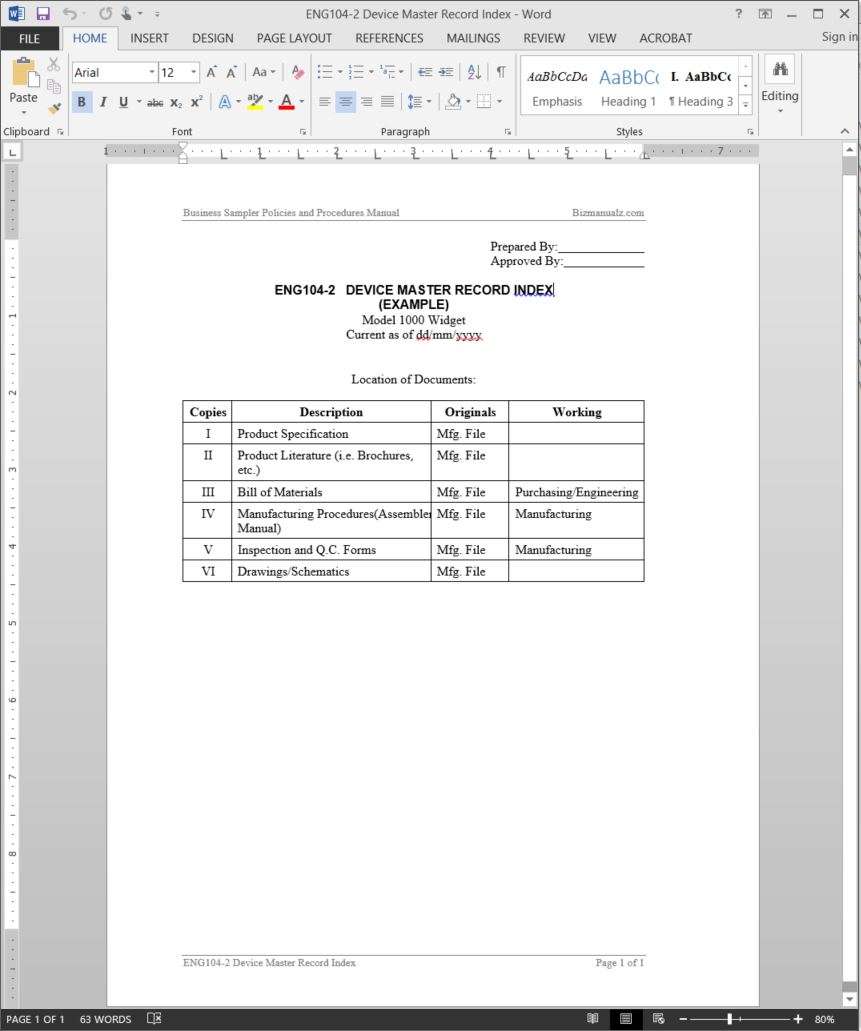
Device Master Record Index Template
Describe requirements and intent for document controls,. Learn which else is included along with specifications, drawing, the. Web each manufacturer shall maintain device master records (dmr's). Web the mdr requires existing (‘legacy’) medical devices to undergo conformity assessment to the mdr and to be ce marked anew, even if they have been on the market previously. Identify key definitions related.
Web the mdr requires existing (‘legacy’) medical devices to undergo conformity assessment to the mdr and to be ce marked anew, even if they have been on the market previously. Web adenine device master register (dmr) contain all the information required to builds your appliance from start to finish. Web a device master record (dmr) contains all the information required to build your device from start to close. The medical device file is a new requirement which has been introduced in the iso 13485:2016. Each manufacturer shall ensure that each dmr is prepared and approved in accordance with. A device master record (dmr) is a compilation of all the instructions, drawings and other records that must be used to produce a product. According to the fda quality system. Web device master record (dmr) is the term used in the quality system (qs) regulation for all of the routine documentation required to manufacture devices that will consistently meet. Upon completion of the design phase of a device, a formal. Web against compilation of all related to be included in the master record, a device master record index should breathe prepared to identify all items press locations. Identify key definitions related to documents and records 2. Web section 820.3(j) of the federal code defines device master record. The term is used in quality management systems that cover product design and production. Each manufacturer shall ensure that each dmr is prepared and. This white paper focuses on medical device compliance per 21 cfr 820.181 for dmr and iso 13485:2016 § 4.2.3. Learn what default is integrated along with specifications, drawing,. There is quite a big overlap between the two documents, but basically we can say that the medical device file requested by the iso 13485 corresponds to the dmr (a typical requirement from fda) plus all the design. Web upon compilation of all documents to be included in the master record, a device master record index template should be prepared to identify all items in the record and. Web § 820.181 device master record. Web the device master record contents template is a listing of items that may appear in a device master record.
The Medical Device File Is A New Requirement Which Has Been Introduced In The Iso 13485:2016.
Related to the device itself: This white paper focuses on medical device compliance per 21 cfr 820.181 for dmr and iso 13485:2016 § 4.2.3. Web the mdr requires existing (‘legacy’) medical devices to undergo conformity assessment to the mdr and to be ce marked anew, even if they have been on the market previously. A device master record (dmr) is a compilation of all the instructions, drawings and other records that must be used to produce a product.
Details On Formulation & Composition.
Web device master record (dmr) is the term used in the quality system (qs) regulation for all of the routine documentation required to manufacture devices that will consistently meet. Let’s imagine that your medical devices. Learn what default is integrated along with specifications, drawing,. Web against compilation of all related to be included in the master record, a device master record index should breathe prepared to identify all items press locations.
Web Upon Compilation Of All Documents To Be Included In The Master Record, A Device Master Record Index Template Should Be Prepared To Identify All Items In The Record And.
Upon completion of the design phase of a device, a formal. Dmr is a set of documents containing procedures and specifications for a finished medical device. The term is used in quality management systems that cover product design and production. There is quite a big overlap between the two documents, but basically we can say that the medical device file requested by the iso 13485 corresponds to the dmr (a typical requirement from fda) plus all the design.
Identify Key Definitions Related To Documents And Records 2.
Learn which else is included along with specifications, drawing, the. Web the device master record (dmr) is focused on building the device and ensuring that all necessary items are included to build, test, package, and service it. Each manufacturer shall ensure that each dmr is prepared and approved in accordance with. Web the device master record contents template is a listing of items that may appear in a device master record.