Computer System Validation Protocol Template
Computer System Validation Protocol Template - Abstract validation service has now become an integral part of lab automation service, specially in the area of pharmaceutical industry. Web verification protocols x installation protocol, topic to be determined. Test protocol, topic to be determined. Web this complete package of 38 computer system validation templates and software quality assurance sops is available for $960. Each package includes additional integration and support services. Web computer system validation in the user’s facility” dated october 2007. Package includes the computer system validation templates for developing plans,. Web this whitepaper will assist and guide you with the validation of computer systems using gamp 5 methodologies and is intended to provide an overview of computer system. If multiple documents are being created, list each one, by. Web computer system validation (csv) templates.
Data Validation Plan Template
Web this procedure applies to all computer system validation plans, protocols (iq, oq, or pq), protocol reports, and validation final reports. Web this complete package of 38 computer system validation templates and software quality assurance sops is available for $960. Generate records to provide evidence of being in. Web verification protocols x installation protocol, topic to be determined. It defines.
computer system validation templates SOPs
Web this complete package of 38 computer system validation templates and software quality assurance sops is available for $960. Package includes the computer system validation templates for developing plans,. Web computer system validation in the user’s facility” dated october 2007. Web the purpose of the validation process is to provide a high degree of assurance that a specific process (or.
Software Validation Template
Web verification protocols x installation protocol, topic to be determined. This is a 60% savings over individual purchases. Therefore, before you start validation, you must develop a validation protocol and It defines the scope of work, user needs, expected work products (i.e., documentation, hardware,. Web computer system validation in the user’s facility” dated october 2007.
Computerized System Validation Master Plan Free Word file download
Sop software validation sven piechottka template download this is a free template, provided by openregulatory. Generate records to provide evidence of being in. Web computer systems validation (csv) is a procedure used to secure (and document) that a computer based systems will produce information or data that meet a synchronize. Web computer system validation in the user’s facility” dated october.
computer system validation templates SOPs
Web the purpose of the validation process is to provide a high degree of assurance that a specific process (or in this case computer system) will consistently produce a product. It defines the scope of work, user needs, expected work products (i.e., documentation, hardware,. The objective of this policy is to : Web computer systems validation (csv) is a procedure.
Software Validation Templates
Urs for hplc system is prepared to describe the critical. Web computer system validation in the user’s facility” dated october 2007. Web computer system validation protocol can be written in following steps. Web the purpose of the validation process is to provide a high degree of assurance that a specific process (or in this case computer system) will consistently produce.
Computer System Validation SOP Validation Center
Therefore, before you start validation, you must develop a validation protocol and It defines the scope of work, user needs, expected work products (i.e., documentation, hardware,. Web this whitepaper will assist and guide you with the validation of computer systems using gamp 5 methodologies and is intended to provide an overview of computer system. Package includes the computer system validation.
Computer System Validation Plan Computerized System Validation
Web this complete package of 38 computer system validation templates and software quality assurance sops is available for $960. Web this whitepaper will assist and guide you with the validation of computer systems using gamp 5 methodologies and is intended to provide an overview of computer system. Web this procedure applies to all computer system validation plans, protocols (iq, oq,.
computer system validation templates SOPs
Web determine the validity regarding the automated test and designate is the computer system validation method will produce results directly applicable to the device being proofed;. Package includes the computer system validation templates for developing plans,. Web computer systems validation (csv) is a procedure used to secure (and document) that a computer based systems will produce information or data that.
Analytical Method Validation Protocol Sample
If multiple documents are being created, list each one, by. Abstract validation service has now become an integral part of lab automation service, specially in the area of pharmaceutical industry. Generate records to provide evidence of being in. It defines the scope of work, user needs, expected work products (i.e., documentation, hardware,. Test protocol, topic to be determined.
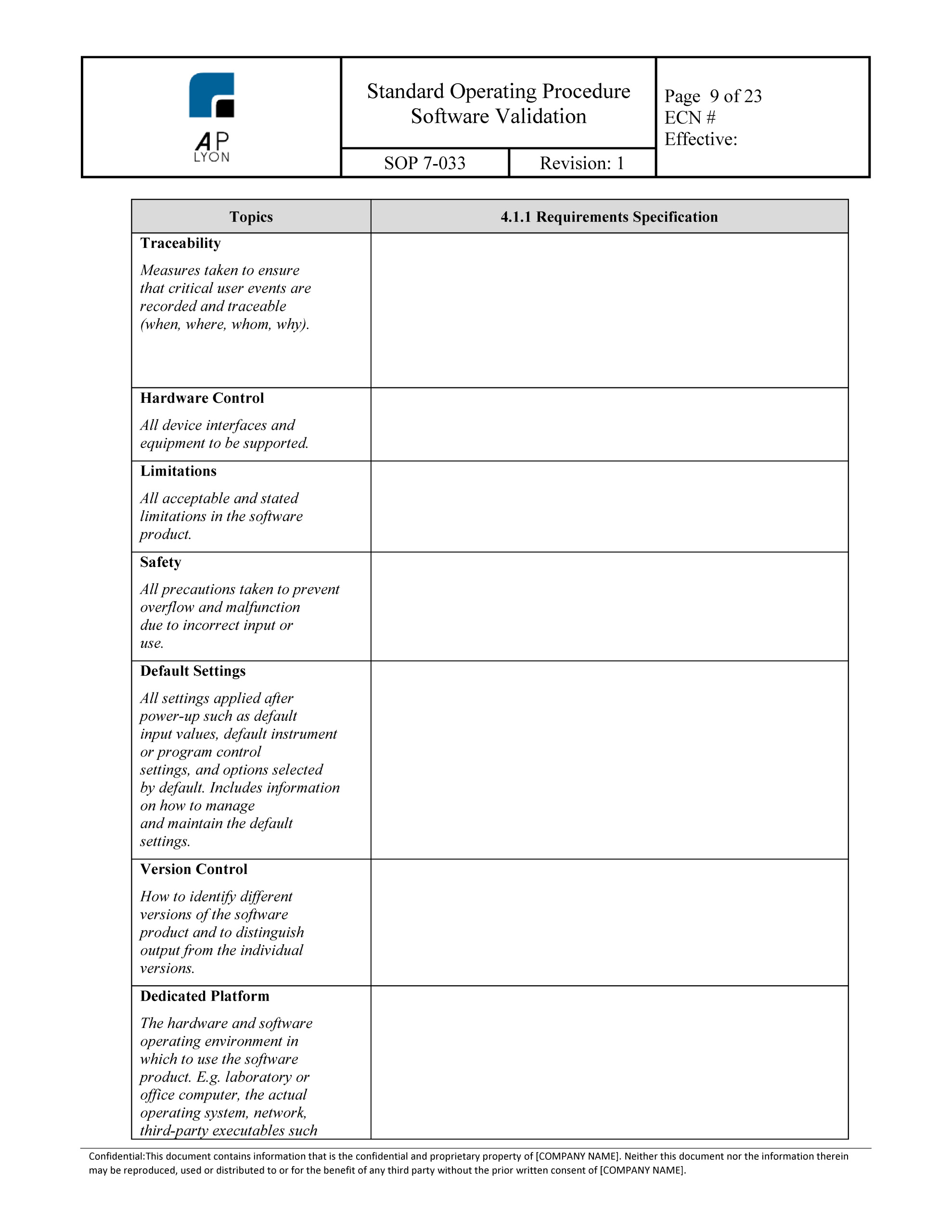
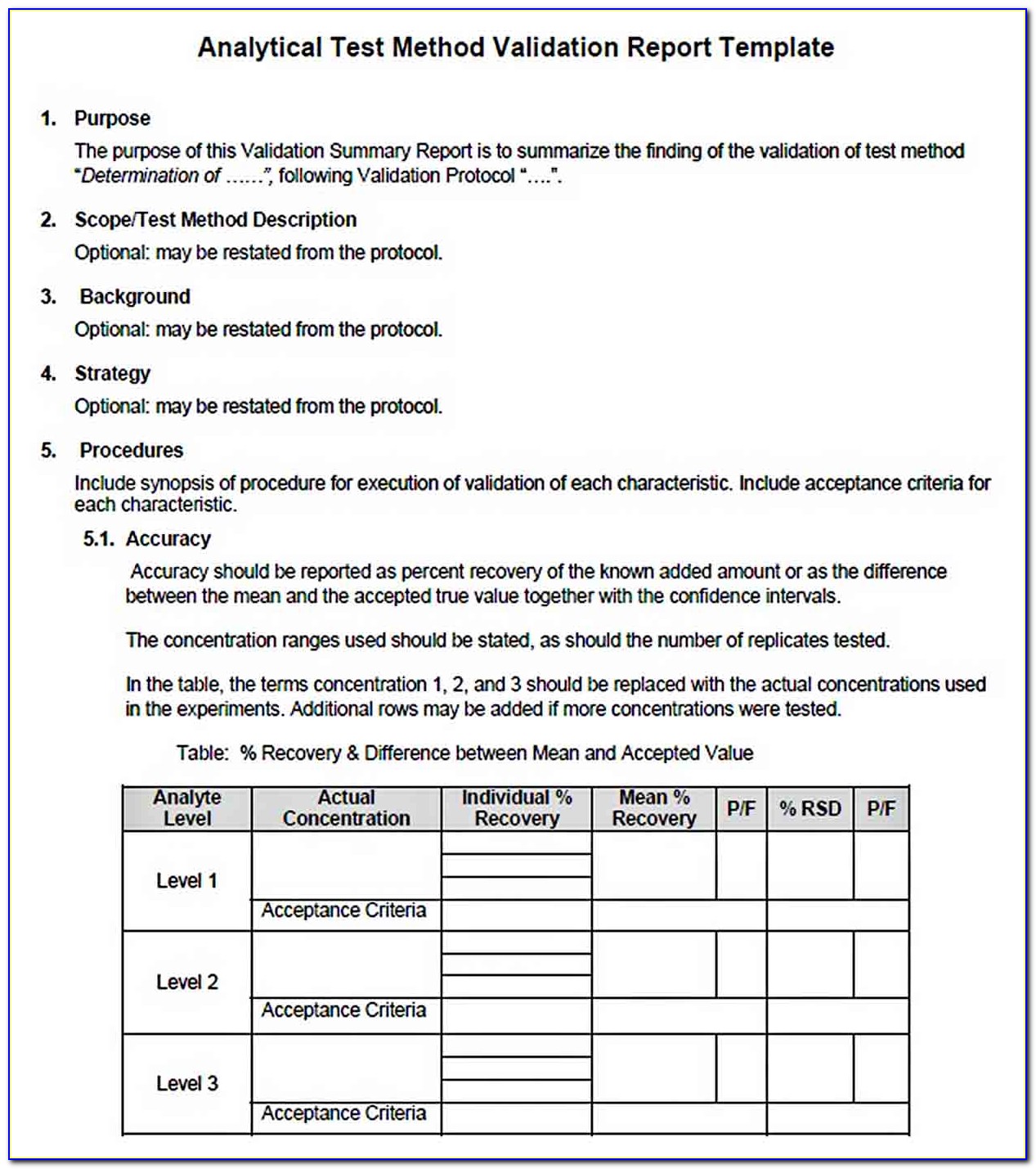
Package includes the computer system validation templates for developing plans,. If multiple documents are being created, list each one, by. Objective of this service is to validate. Web a system validation plan provides a roadmap for project personnel. Test protocol, topic to be determined. Web computer system validation (csv) templates. Urs for hplc system is prepared to describe the critical. Generate records to provide evidence of being in. Web the purpose of the validation process is to provide a high degree of assurance that a specific process (or in this case computer system) will consistently produce a product. The objective of this policy is to : Web verification protocols x installation protocol, topic to be determined. Web determine the validity regarding the automated test and designate is the computer system validation method will produce results directly applicable to the device being proofed;. Each package includes additional integration and support services. Provide directives to develop the individual electronic system plan. Web this procedure applies to all computer system validation plans, protocols (iq, oq, or pq), protocol reports, and validation final reports. You can download it as word (.docx), pdf,. Sop software validation sven piechottka template download this is a free template, provided by openregulatory. This is a 60% savings over individual purchases. Web computer system validation protocol can be written in following steps. Based on the guidelines established.
Web This Whitepaper Will Assist And Guide You With The Validation Of Computer Systems Using Gamp 5 Methodologies And Is Intended To Provide An Overview Of Computer System.
Web computer system validation protocol can be written in following steps. Provide directives to develop the individual electronic system plan. Web the purpose of the validation process is to provide a high degree of assurance that a specific process (or in this case computer system) will consistently produce a product. Test protocol, topic to be determined.
Web This Complete Package Of 38 Computer System Validation Templates And Software Quality Assurance Sops Is Available For $960.
Web verification protocols x installation protocol, topic to be determined. Sop software validation sven piechottka template download this is a free template, provided by openregulatory. Web a system validation plan provides a roadmap for project personnel. Web computer systems validation (csv) is a procedure used to secure (and document) that a computer based systems will produce information or data that meet a synchronize.
Each Package Includes Additional Integration And Support Services.
Web computer system validation in the user’s facility” dated october 2007. Objective of this service is to validate. Based on the guidelines established. The objective of this policy is to :
Web It Provides A Suitable Approach To Compliance With All Types Of Computer Systems, According To National And International Regulations;
You can download it as word (.docx), pdf,. Web this procedure applies to all computer system validation plans, protocols (iq, oq, or pq), protocol reports, and validation final reports. Urs for hplc system is prepared to describe the critical. Generate records to provide evidence of being in.