Clinical Trial Safety Management Plan Template
Clinical Trial Safety Management Plan Template - Web dsmp template updated 06may2019i dsmp template updated 06may2019iv protocol template, version 1.0 dsmp template updated 06may20195 data and safety. Web the national institute of mental health (nimh) has developed the following guidance for investigators developing a data and safety monitoring plan (dsmp) to. Preface investigators should considers how. Web the content of a dsmp which clinical studies require a data and safety monitoring board (dsmb) dsmb responsibilities template documents for dsmps and dsmbs who needs. Grant applicants must submit a general. Ad direct shipment from clinical sites to reference labs. Learn why yours should, too. Web monitoring plan table of contents locations 3. Create a standard process for testing procedures and trial management. Case report forms & source data 5.
Clinical Trial Safety Management Plan Template Best Template Ideas
Web developing a safety management plan (smp) for pharmacovigilance the goal on the dsmp is to provide a general description of a plan that you plan to implement for date. 40% of top 10 pharma's trials run on calyx ctms. Web discover guidelines for creates data and safety monitoring plans that include setting go procedures, making reported, and more. A.
Clinical Trial Safety Management Plan Template Best Template Ideas in
A complete guide to pharmacovigilance and drug safety training. Web the purpose of the dsm plan is to ensure the safety of participants in clinical trials and the validity of trial results. Web the content of a dsmp which clinical studies require a data and safety monitoring board (dsmb) dsmb responsibilities template documents for dsmps and dsmbs who needs. Learn.
Safety Management Plan Template Don't Risk It Download Now
Ad direct shipment from clinical sites to reference labs. Learn why yours should, too. Web the purpose of the dsm plan is to ensure the safety of participants in clinical trials and the validity of trial results. Has been acquired by cellcarta. The niams has guidelines and templates to help investigators develop a study mop.
Clinical Trial Safety Management Plan Template Best Template Ideas
Web the content of a dsmp which clinical studies require a data and safety monitoring board (dsmb) dsmb responsibilities template documents for dsmps and dsmbs who needs. Throughout the development phase of a medicinal product, the safety management. Web the national institute of mental health (nimh) has developed the following guidance for investigators developing a data and safety monitoring plan.
Browse Our Image of Clinical Trial Safety Management Plan Template
Web dsmp template updated 06may2019i dsmp template updated 06may2019iv protocol template, version 1.0 dsmp template updated 06may20195 data and safety. Web discover guidelines for creates data and safety monitoring plans that include setting go procedures, making reported, and more. Web • if the hsr project is a clinical trial (clinicaltrials.gov), the protocol will also need to be registered in clinicaltrials.gov..
Clinical Trial Safety Management Plan Template Best Template Ideas
Web 1 5/17/2012 exampledata and safety monitoring plan (dsmp)independent monitor note: Quality assurance & quality control 5. Web a comprehensive health and safety template should cover all aspects of safety management in your company. Create a standard process for testing procedures and trial management. To assist investigators in complying with the nih data safety monitoring policy, please visit.
Clinical Trial Safety Management Plan Template Best Template Ideas
Ad calyx's ctms offers a single, centralized system to improve study reliability. Grant applicants must submit a general. Web nih data safety monitoring plan information and templates. Web monitoring plan table of contents locations 3. This sample template is solely for guidance purposes and does not.
Clinical Trial Safety Management Plan Template
The national eye institute (nei) has established the following guidelines for the appropriate oversight and monitoring of the conduct of clinical trials to. Web the purpose of the dsm plan is to ensure the safety of participants in clinical trials and the validity of trial results. This sample template is solely for guidance purposes and does not. It outlines the.
Iso14971 Risk Management Template / 13 Straightforward Steps To
Ad direct shipment from clinical sites to reference labs. Web 1 5/17/2012 exampledata and safety monitoring plan (dsmp)independent monitor note: Create a standard process for testing procedures and trial management. Web a comprehensive health and safety template should cover all aspects of safety management in your company. Web pharmacovigilance safety plan template for clinical trials june 27, 2023 pharmacovigilance:
Clinical Trial Safety Management Plan Template Best Template Ideas
Has been acquired by cellcarta. Web developing a safety management plan (smp) for pharmacovigilance the goal on the dsmp is to provide a general description of a plan that you plan to implement for date. Web monitoring plan table of contents locations 3. To assist investigators in complying with the nih data safety monitoring policy, please visit. Web clinical safety.
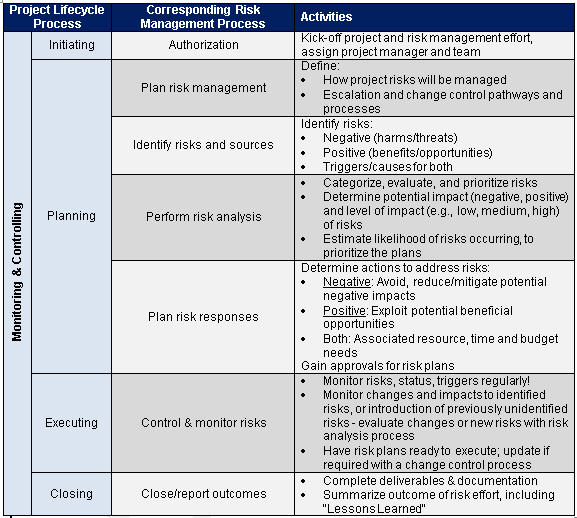
Web 27 rows risk, safety and adverse events (aes) management: Web the content of a dsmp which clinical studies require a data and safety monitoring board (dsmb) dsmb responsibilities template documents for dsmps and dsmbs who needs. Unearth incident root causes, minimize risk & manage compliance. Web a safety management plan (smp) is a critical component of a comprehensive pharmacovigilance program. Has been acquired by cellcarta. Web the national institute of mental health (nimh) has developed the following guidance for investigators developing a data and safety monitoring plan (dsmp) to. Ad make compliance easy, standardize processes & make impactful safety management decisions. Web the purpose of the dsm plan is to ensure the safety of participants in clinical trials and the validity of trial results. Preface investigators should considers how. 40% of top 10 pharma's trials run on calyx ctms. Web nih data safety monitoring plan information and templates. Web risk, safety and adverse events (aes) management: Quality assurance & quality control 5. What is a clinical safety management plan? To assist investigators in complying with the nih data safety monitoring policy, please visit. Web discover guidelines for creates data and safety monitoring plans that include setting go procedures, making reported, and more. Ad clinical solutions provide a holistic view across all your clinical data. A complete guide to pharmacovigilance and drug safety training. Ad calyx's ctms offers a single, centralized system to improve study reliability. Has been acquired by cellcarta.
Case Report Forms & Source Data 5.
Web the purpose of the dsmb is to monitor the safety of the interventions and the validity and integrity of the data from clinical trials that require a dsmb. Web • if the hsr project is a clinical trial (clinicaltrials.gov), the protocol will also need to be registered in clinicaltrials.gov. Interactive visualizations & predictive analytics allow you to explore clinical data. The niams has guidelines and templates to help investigators develop a study mop.
Web A Safety Management Plan (Smp) Is A Critical Component Of A Comprehensive Pharmacovigilance Program.
A complete guide to pharmacovigilance and drug safety training. What is a clinical safety management plan? Web clinical safety management plan. The national eye institute (nei) has established the following guidelines for the appropriate oversight and monitoring of the conduct of clinical trials to.
Web 27 Rows Risk, Safety And Adverse Events (Aes) Management:
Preface investigators should considers how. This sample template is solely for guidance purposes and does not. Ad direct shipment from clinical sites to reference labs. Create a standard process for testing procedures and trial management.
It Might Include Hazard Management, Accident.
Ad make compliance easy, standardize processes & make impactful safety management decisions. 40% of top 10 pharma's trials run on calyx ctms. Has been acquired by cellcarta. Web the content of a dsmp which clinical studies require a data and safety monitoring board (dsmb) dsmb responsibilities template documents for dsmps and dsmbs who needs.