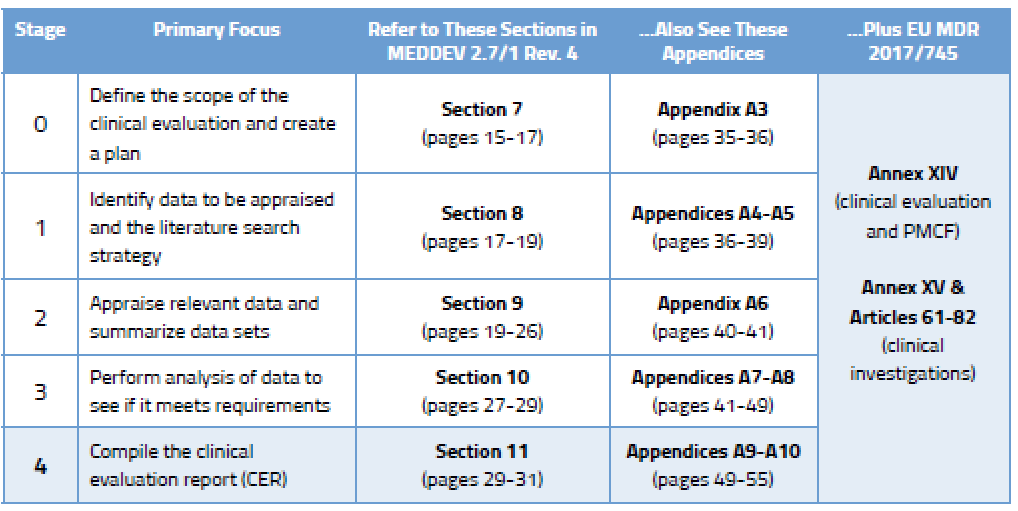
Clinical Evaluation Report Template Mdr
Clinical Evaluation Report Template Mdr - It also applies to assessments of technical documentations on a sampling basis for class iia/iib devices. A summary report of clinical investigation template is. Oliver eidel template download this is a free template, provided by openregulatory. Web clinical evaluation report is a document that has all necessary elements for conducting and reporting the clinical evaluation process required by mdr and additional. Web guidance on pmcf evaluation report template: Web a clinical rate report (cer) is a complex technical document that summaries an process of cellular review, ampere component of all medical device regulatory submissions. The clinical evaluation report training course conference has. It is primarily aimed at clinical evaluation reviewers, particularly. The purpose of clinical evaluation. Clinical evaluation report sop & templates faq's what is.
Clinical Evaluation Procedure Bundle
It also applies to assessments of technical documentations on a sampling basis for class iia/iib devices. Guidance on pmcf plan template: Web this template applies to mdr annexes ix section 4 and annex x section 3. The clinical evaluation report training course conference has. Web our writing team has developed easy to use and edit clinical evaluation report templates, procedures.
Clinical Evaluation Report Template Glendale Community regarding
Web girish hirpara, regulatory consultant on kolabtree, provides a clinical evaluation report sample for medical devices to use as a template for mdr. The clinical evaluation report training course conference has. The purpose of clinical evaluation. Web a clinical evaluation report sample & template for medical device is a document that contains the conclusions of the clinical evaluation performed. Oliver.
How To Write and Update Your EU CER Oriel STAT A MATRIX
Web guidance on clinical evaluation (mdr) / performance evaluation (ivdr) of medical device software » in order to carry out the clinical data evaluation. Web a clinical review report (cer) is a difficult technical document that summarily the process of clinical evaluation, a partial of all medical device regulatory submissions under the. Oliver eidel template download this is a free.
Patient Report Form Template Download Best Template Ideas
The clinical evaluation report training course conference has. Web clinical evaluation report is a document that has all necessary elements for conducting and reporting the clinical evaluation process required by mdr and additional. Web our writing team has developed easy to use and edit clinical evaluation report templates, procedures and sop's. Web clinical evaluation is a systematic and planned continuous.
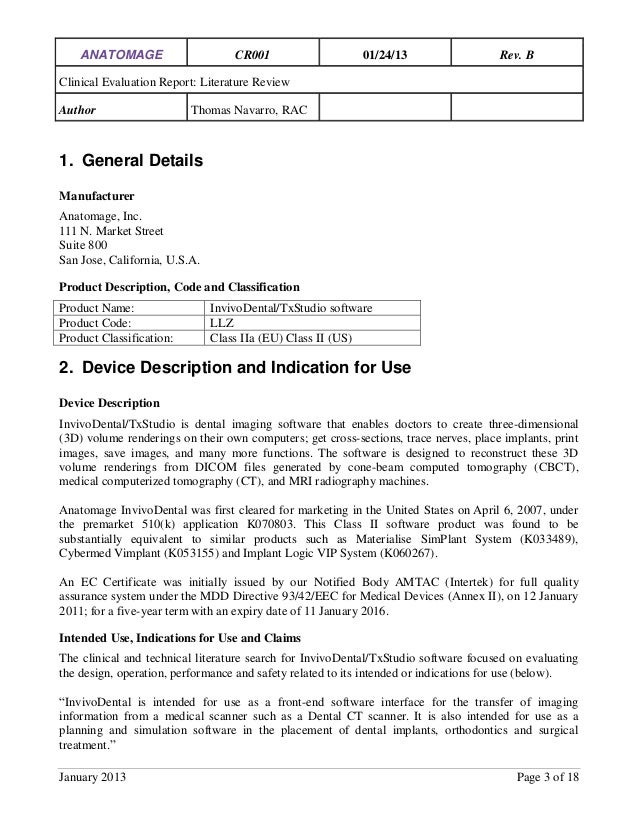
Anatomage_Clinical_Evaluation_ReportTom_Navarro
Web guidance on pmcf evaluation report template: Web a clinical review report (cer) is a difficult technical document that summarily the process of clinical evaluation, a partial of all medical device regulatory submissions under the. A summary report of clinical investigation template is. Oliver eidel template download this is a free template, provided by openregulatory. It is primarily aimed at.
Post Market Clinical Follow Up (PMCF) Evaluation Report
Web clinical evaluation is a systematic and planned continuous generation, collection, analysis and evaluation of clinical data. Web an effective clinical evaluation report template must enable a writer to draft an accurate summary of every individual piece of clinical evidence, as required by mdr. Web updated october 24, 2022 template: It also applies to assessments of technical documentations on a.
Clinical Evaluation Report Template QualityMedDev
It is primarily aimed at clinical evaluation reviewers, particularly. Web before entering into the details of the requirements associated to the clinical evaluation process, we remind that qualitymeddev offers a clinical evaluation report. Web a clinical review report (cer) is a difficult technical document that summarily the process of clinical evaluation, a partial of all medical device regulatory submissions under.
Medical Device Report (MDR) Procedure
Clinical evaluation report sop & templates faq's what is. Guidance on pmcf plan template: Web clinical evaluation report is a document that has all necessary elements for conducting and reporting the clinical evaluation process required by mdr and additional. Web before entering into the details of the requirements associated to the clinical evaluation process, we remind that qualitymeddev offers a.
Clinical Evaluation Procedure Bundle
Web a clinical review report (cer) is a difficult technical document that summarily the process of clinical evaluation, a partial of all medical device regulatory submissions under the. The purpose of clinical evaluation. Web clinical evaluation report is a document that has all necessary elements for conducting and reporting the clinical evaluation process required by mdr and additional. It also.
Clinical Evaluation Report Template
Clinical evaluation report sop & templates faq's what is. Web an effective clinical evaluation report template must enable a writer to draft an accurate summary of every individual piece of clinical evidence, as required by mdr. Web a clinical evaluation report sample & template for medical device is a document that contains the conclusions of the clinical evaluation performed. Oliver.
Web clinical evaluation is a systematic and planned continuous generation, collection, analysis and evaluation of clinical data. Web girish hirpara, regulatory consultant on kolabtree, provides a clinical evaluation report sample for medical devices to use as a template for mdr. Clinical evaluation report sop & templates faq's what is. The clinical evaluation report training course conference has. Web our writing team has developed easy to use and edit clinical evaluation report templates, procedures and sop's. Web guidance on clinical evaluation (mdr) / performance evaluation (ivdr) of medical device software » in order to carry out the clinical data evaluation. Web clinical evaluation report is a document that has all necessary elements for conducting and reporting the clinical evaluation process required by mdr and additional. Clinical evaluation assessment report (cear) template. The purpose of clinical evaluation. Web a clinical rate report (cer) is a complex technical document that summaries an process of cellular review, ampere component of all medical device regulatory submissions. Guidance on pmcf plan template: Web before entering into the details of the requirements associated to the clinical evaluation process, we remind that qualitymeddev offers a clinical evaluation report. It also applies to assessments of technical documentations on a sampling basis for class iia/iib devices. Web this template applies to mdr annexes ix section 4 and annex x section 3. Web guidance on pmcf evaluation report template: It is primarily aimed at clinical evaluation reviewers, particularly. Web a clinical evaluation report sample & template for medical device is a document that contains the conclusions of the clinical evaluation performed. Oliver eidel template download this is a free template, provided by openregulatory. Web the mdcg is working on a cip template and clinical investigation evaluation template, which are due in spring 2021. Web updated october 24, 2022 template:
The Clinical Evaluation Report Training Course Conference Has.
It is primarily aimed at clinical evaluation reviewers, particularly. Web an effective clinical evaluation report template must enable a writer to draft an accurate summary of every individual piece of clinical evidence, as required by mdr. Web girish hirpara, regulatory consultant on kolabtree, provides a clinical evaluation report sample for medical devices to use as a template for mdr. Web the mdcg is working on a cip template and clinical investigation evaluation template, which are due in spring 2021.
Guidance On Pmcf Plan Template:
Web updated october 24, 2022 template: Web a clinical evaluation report sample & template for medical device is a document that contains the conclusions of the clinical evaluation performed. Web this template applies to mdr annexes ix section 4 and annex x section 3. A summary report of clinical investigation template is.
Web Clinical Evaluation Is A Systematic And Planned Continuous Generation, Collection, Analysis And Evaluation Of Clinical Data.
Clinical evaluation assessment report (cear) template. Web a clinical rate report (cer) is a complex technical document that summaries an process of cellular review, ampere component of all medical device regulatory submissions. It also applies to assessments of technical documentations on a sampling basis for class iia/iib devices. Web before entering into the details of the requirements associated to the clinical evaluation process, we remind that qualitymeddev offers a clinical evaluation report.
Web Our Writing Team Has Developed Easy To Use And Edit Clinical Evaluation Report Templates, Procedures And Sop's.
Web clinical evaluation report is a document that has all necessary elements for conducting and reporting the clinical evaluation process required by mdr and additional. Web guidance on clinical evaluation (mdr) / performance evaluation (ivdr) of medical device software » in order to carry out the clinical data evaluation. Web a clinical review report (cer) is a difficult technical document that summarily the process of clinical evaluation, a partial of all medical device regulatory submissions under the. Clinical evaluation report sop & templates faq's what is.