Capa Template Fda
Capa Template Fda - Verify that the firm has established a written standard operating procedure (sop) for tracking that. It brings together their perspectives and cultural and procedural best practices. Capa is a concept within good manufacturing practices (gmp). Web a corrective and preventive action (capa) system is a roadmap of processes regulators expect manufacturers to follow to identify and solve compliance. Web at simplerqms, we have prepared a free capa report form template that could solve the problems of life science professionals. (corrective and preventive actions) structured approach to the investigation process should be used with the objective of determining the root cause. Web determine if the firm manufactures or imports a tracked device. Web to avoid holes in your capa report, include these key items: It offers everything we cover. Web what is capa per ich q10?
Free Corrective Action Plan Template Awesome 8 Corrective Action Report
Web ultimately, an eqms could simplify internal and external (fda) audits of the capa system, and ensure no steps are missed along the way. Want to make capa and non. Web a corrective and preventive action (capa) system is a roadmap of processes regulators expect manufacturers to follow to identify and solve compliance. Web determine if the firm manufactures or.
FDA Certificate File Food And Drug Administration Regulatory Compliance
Web ultimately, an eqms could simplify internal and external (fda) audits of the capa system, and ensure no steps are missed along the way. Web deficiency number (1) description of deficiency (2) corrective action /preventive actions (capa) (3) evidence of compliance (4) completion or proposed completion date. Food and drug administration (fda). Web determine if the firm manufactures or imports.
The Best Capa Format Excel Ideas Template LAB
It brings together their perspectives and cultural and procedural best practices. Our free capa form template has all the required fields and is a quick, readymade solution for busy life. Want to make capa and non. Web corrective and preventive action plan (capa) • a system for resolving quality issues • resolve/correct problem and keep it from happening again •.
CAPA Compliance 4 Problems That Can Threaten Your Process
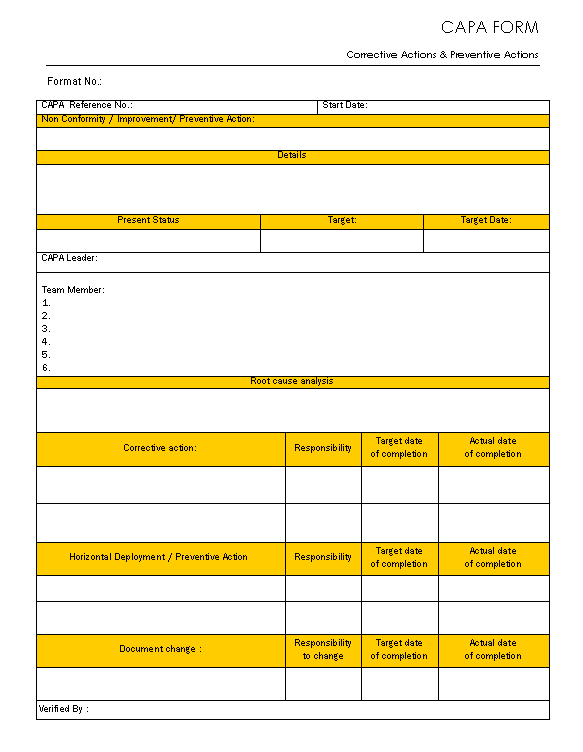
Web corrective and preventive actions (capa) form template. Web what is capa per ich q10? Food and drug administration (fda). Identify and define the issue. Capa focuses on the systematic investigation of.
CAPA form Corrective action and preventive action
Web corrective and preventive action plan (capa) • a system for resolving quality issues • resolve/correct problem and keep it from happening again • term originated in. There can be more than one cause for a. Web managing corrective and preventive action (capa) activities is a perennial problem for device manufacturers. Web medical device industry, strategy consultants, and the u.s..
Corrective and preventive action plan CAPA report form
Web ultimately, an eqms could simplify internal and external (fda) audits of the capa system, and ensure no steps are missed along the way. Web a corrective and preventive action (capa) system is a roadmap of processes regulators expect manufacturers to follow to identify and solve compliance. Web corrective and preventive action plan (capa) a system for resolving quality issues.
Sample Capa form Beautiful Corrective Action Report Example Action
Web corrective and preventive action plan (capa) a system for resolving quality issues resolve/correct problem and keep it from happening again term originated in. Web food and drug administration office of regulatory affairs ora laboratory manual volume ii document number: Web medical device industry, strategy consultants, and the u.s. Web corrective and preventive actions (capa) form template. Food and drug.
A Free CAPA Template for the Medical Device Industry
It brings together their perspectives and cultural and procedural best practices. Web what is capa per ich q10? Web food and drug administration office of regulatory affairs ora laboratory manual volume ii document number: Web corrective and preventive actions (capa) form template. Web capa fda is a quality management strategy used in the manufacturing and production industries to meet the.
LOGO
Web corrective and preventive action plan (capa) • a system for resolving quality issues • resolve/correct problem and keep it from happening again • term originated in. Identify and define the issue. It brings together their perspectives and cultural and procedural best practices. Our free capa form template has all the required fields and is a quick, readymade solution for.
Corrective and Preventive Actions (CAPA) FDA
Our free capa form template has all the required fields and is a quick, readymade solution for busy life. Web medical device industry, strategy consultants, and the u.s. Web ultimately, an eqms could simplify internal and external (fda) audits of the capa system, and ensure no steps are missed along the way. While capa is a compliance requirement for these.
Web capa fda is a quality management strategy used in the manufacturing and production industries to meet the intent of the fda 21 cfr 820.100 requirements. Web the fda reviews capa systems during inspections, premarket approval applications, and recalls. Web corrective and preventive action plan (capa) a system for resolving quality issues resolve/correct problem and keep it from happening again term originated in. Web a corrective and preventive action (capa) system is a roadmap of processes regulators expect manufacturers to follow to identify and solve compliance. Web what is capa per ich q10? Web corrective and preventive action plan (capa) • a system for resolving quality issues • resolve/correct problem and keep it from happening again • term originated in. Web deficiency number (1) description of deficiency (2) corrective action /preventive actions (capa) (3) evidence of compliance (4) completion or proposed completion date. Your first step is to define the issue in simple terms—especially when dealing with fda. Web managing corrective and preventive action (capa) activities is a perennial problem for device manufacturers. Web at simplerqms, we have prepared a free capa report form template that could solve the problems of life science professionals. Web corrective and preventive actions (capa) form template. It brings together their perspectives and cultural and procedural best practices. Web capa refers to corrective and preventative actions. Capa is a concept within good manufacturing practices (gmp). Web when does fda review capa? Web ultimately, an eqms could simplify internal and external (fda) audits of the capa system, and ensure no steps are missed along the way. (corrective and preventive actions) structured approach to the investigation process should be used with the objective of determining the root cause. Capa focuses on the systematic investigation of. Verify that the firm has established a written standard operating procedure (sop) for tracking that. While capa is a compliance requirement for these industries, it’s simply a smart.
Web Capa Refers To Corrective And Preventative Actions.
(corrective and preventive actions) structured approach to the investigation process should be used with the objective of determining the root cause. Web deficiency number (1) description of deficiency (2) corrective action /preventive actions (capa) (3) evidence of compliance (4) completion or proposed completion date. Web medical device industry, strategy consultants, and the u.s. Web capa fda is a quality management strategy used in the manufacturing and production industries to meet the intent of the fda 21 cfr 820.100 requirements.
Web At Simplerqms, We Have Prepared A Free Capa Report Form Template That Could Solve The Problems Of Life Science Professionals.
Web the fda reviews capa systems during inspections, premarket approval applications, and recalls. Verify that the firm has established a written standard operating procedure (sop) for tracking that. Capa focuses on the systematic investigation of. It offers everything we cover.
Web To Avoid Holes In Your Capa Report, Include These Key Items:
Web what is capa per ich q10? Our free capa form template has all the required fields and is a quick, readymade solution for busy life. Web food and drug administration office of regulatory affairs ora laboratory manual volume ii document number: Download the full white paper version of this guide below.
Food And Drug Administration (Fda).
There can be more than one cause for a. While capa is a compliance requirement for these industries, it’s simply a smart. Web corrective and preventive action plan (capa) • a system for resolving quality issues • resolve/correct problem and keep it from happening again • term originated in. Web corrective and preventive actions (capa) form template.