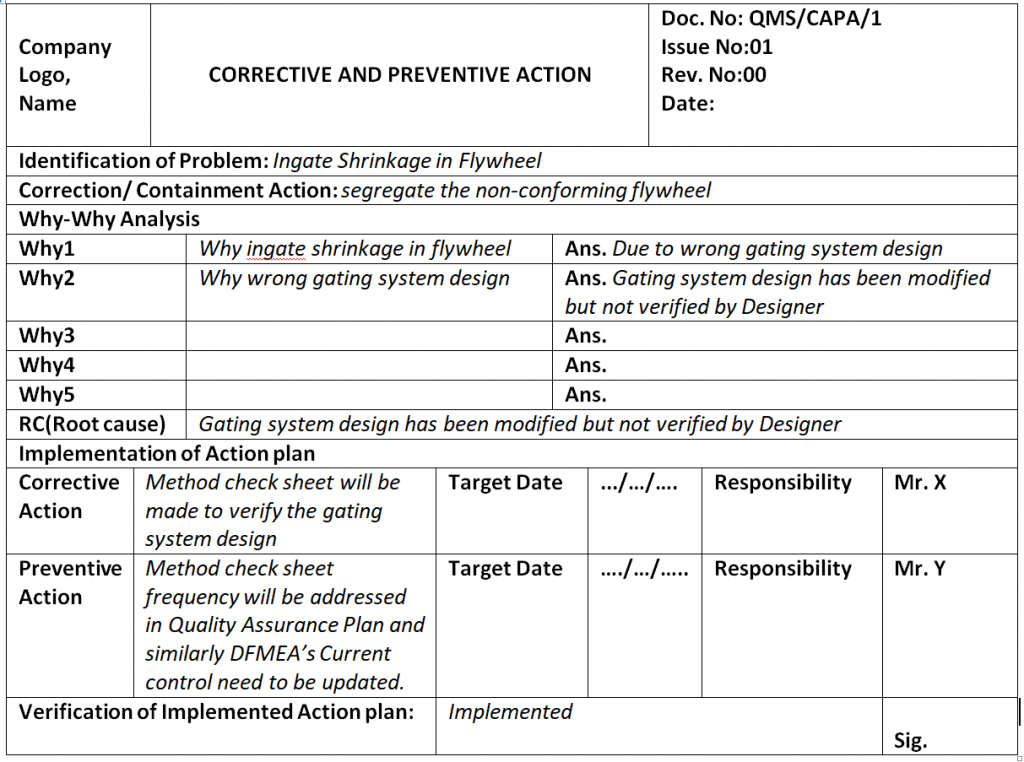
Capa Template Clinical Research
Capa Template Clinical Research - Understand what findings require an action plan vs. Web learn about capa — corrective actions and preventive actions — and how its processes seek out and sustain safety and quality across multiple industries. Corrective and preven tative action (capa) p lan last revised:. Let us take a look at five capa best practices that can strengthen clinical research compliance and also help accomplish a fundamental goal. Web capas in clinical research fda guidance: It will cover when and why capas are. Corrective and preventive actions (capa) plans. Web standard operating procedures (sop) for clinical research title: Discuss all research procedures involved in the human research for each subject population participating in the study. Easy to use, instant deployment.
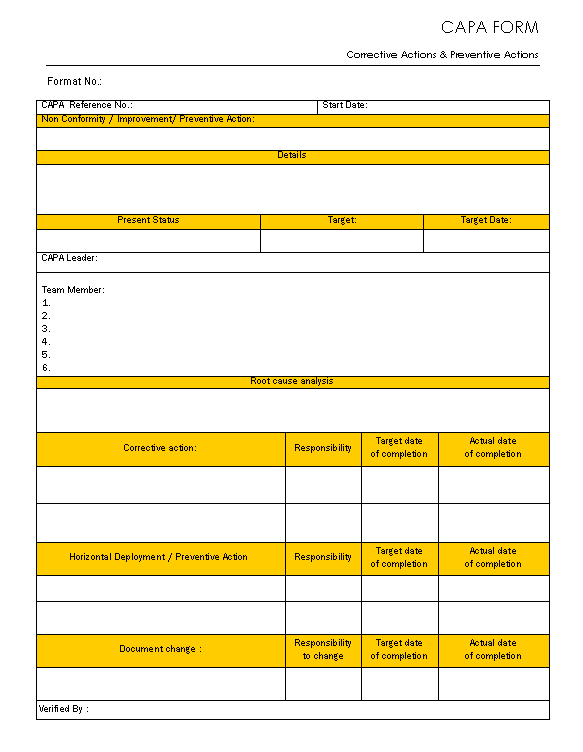
Sample Capa form Peterainsworth
Web standard operating procedures (sop) for clinical research title: Web learn about capa — corrective actions and preventive actions — and how its processes seek out and sustain safety and quality across multiple industries. The first step in developing a capa plan is to identify. Guiding clinical research professionals in improving weaknesses, deficiencies, or in rectifying. Web capa is written.
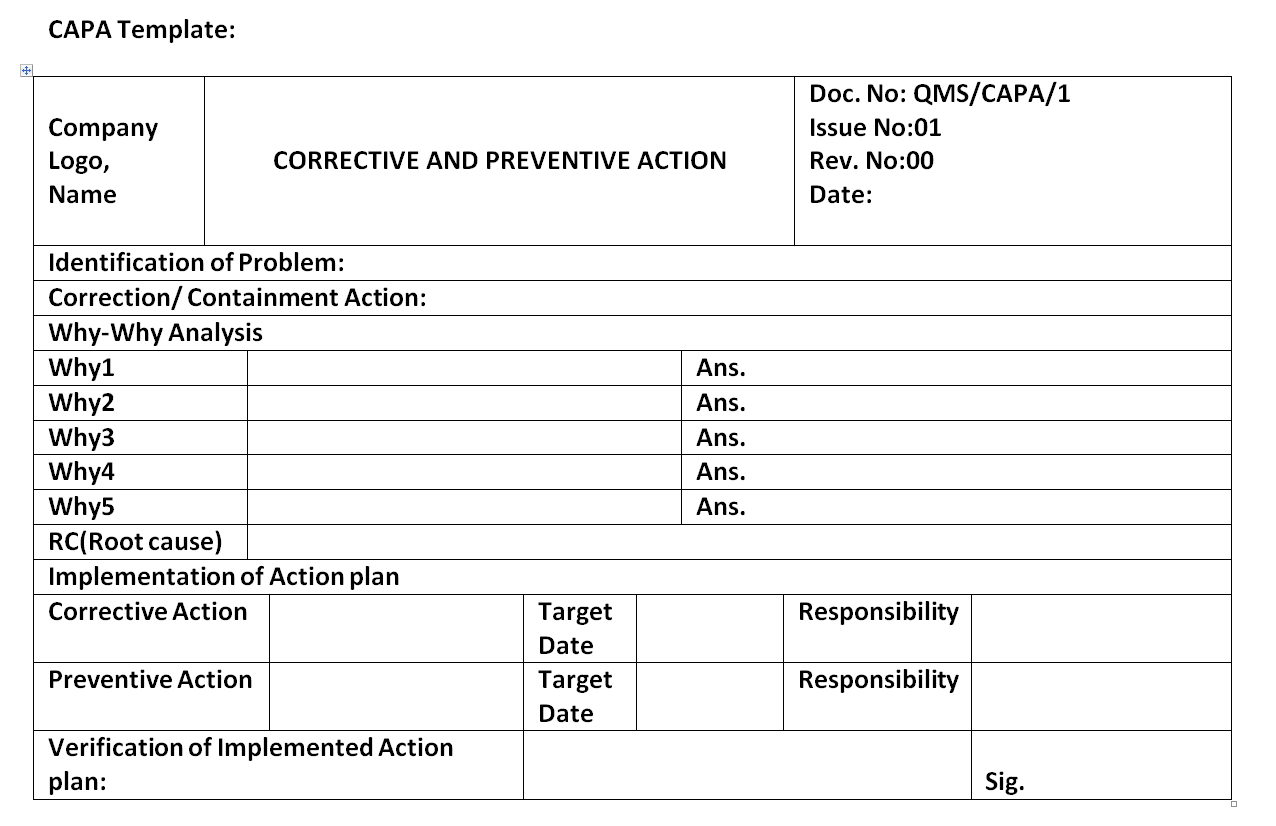
Howtocreate capa template
Discuss all research procedures involved in the human research for each subject population participating in the study. •your capa might have to involve other studies under the pi and/or using the same study staff. Web include how long subject participation is expected to last. Web capa is written to identify a discrepancy/problem in the conduct of a clinical research study,.
15+ Capa Vorlage MelTemplates MelTemplates
Guiding clinical research professionals in improving weaknesses, deficiencies, or in rectifying. Download corrective and preventative action plan form template_2019.11.13. Identify common findings found in research study reviews conducted by the ctqa program. Web the iu hrpp quality improvement office (qio) has developed a capa plan template to assist study teams with this activity. Guiding clinical research professionals in improving weaknesses,.
Capa Mock Sop Clinical Trial Medicine
Identify common findings found in research study reviews conducted by the ctqa program. It will cover when and why capas are. Track and manage quality events. Guiding clinical research professionals in improving weaknesses, deficiencies, or in rectifying. Corrective and preven tative action (capa) p lan last revised:.
CAPA form Corrective action and preventive action
Web corrective and preventive actions (capa) plans. Web corrective and preventive action (capa) plans. Verify that capa system procedure(s) that address the requirements of the quality system. Download corrective and preventative action plan form template_2019.11.13. Web capas can be used for audit or inspection observations, compliance improvement, or risk mitigation.
A Free CAPA Template for the Medical Device Industry
Download corrective and preventative action plan form template_2019.11.13. Web capa is written to identify a discrepancy/problem in the conduct of a clinical research study, note the root cause of the identified problem, identify the corrective action to. Web learn about capa — corrective actions and preventive actions — and how its processes seek out and sustain safety and quality across.
PPT Ensuring Quality in Medical Device Clinical Trials PowerPoint
The fda indicates that corrective and preventive actions (capas) are absolutely necessary to resolve problems and. Web include how long subject participation is expected to last. This template is designed to. Web clinical research is the catalyst for bringing groundbreaking scientific discoveries to the forefront in finding new ways to treat, prevent and detect illnesses. Guiding clinical research professionals in.
Corrective and Preventive Action Format CAPA with Example Download
Guiding clinical research professionals in improving weaknesses, deficiencies, or in rectifying deviation patterns and areas of. Web this article provides an overview of the root cause of these problems and how to ensure that corrective and preventive actions are addressing the actual problem. The international conference for harmonization (ich) released. Web include how long subject participation is expected to last..
Capa Report Template Luxury 8d Report On Capa Item in 2020 Report
Corrective and preventive actions (capa) plans. Let us take a look at five capa best practices that can strengthen clinical research compliance and also help accomplish a fundamental goal. Corrective and preven tative action (capa) p lan last revised:. Track and manage quality events. Web corrective and preventive actions (capa) plans.
Clinical Trial Report Template (1) TEMPLATES EXAMPLE TEMPLATES
Web corrective action and preventive action (capa) plan template. Guiding clinical research professionals in improving weaknesses, deficiencies, or in rectifying. Web clinical research is the catalyst for bringing groundbreaking scientific discoveries to the forefront in finding new ways to treat, prevent and detect illnesses. Corrective and preventive actions (capa) plans. Web capas can be used for audit or inspection observations,.
Web capas in clinical research fda guidance: The first step in developing a capa plan is to identify. Web the iu hrpp quality improvement office (qio) has developed a capa plan template to assist study teams with this activity. The fda indicates that corrective and preventive actions (capas) are absolutely necessary to resolve problems and. Web clinical capa process, managing clinical quality using corrective and preventative actions is not new to clinical. Let us take a look at five capa best practices that can strengthen clinical research compliance and also help accomplish a fundamental goal. Web corrective action and preventive action (capa) plan template. Identify common findings found in research study reviews conducted by the ctqa program. Discuss all research procedures involved in the human research for each subject population participating in the study. Web five best practices. Easy to use, instant deployment. It will cover when and why capas are. Corrective and preventive actions (capa) plans. Download corrective and preventative action plan form template_2019.11.13. •your capa might have to involve other studies under the pi and/or using the same study staff. Understand what findings require an action plan vs. Guiding clinical research professionals in improving weaknesses, deficiencies, or in rectifying. Web include how long subject participation is expected to last. This template is designed to. Web capa is written to identify a discrepancy/problem in the conduct of a clinical research study, note the root cause of the identified problem, identify the corrective action to.
Corrective And Preven Tative Action (Capa) P Lan Last Revised:.
Easy to use, instant deployment. Web learn about capa — corrective actions and preventive actions — and how its processes seek out and sustain safety and quality across multiple industries. This template is designed to. Web include how long subject participation is expected to last.
Web The Iu Hrpp Quality Improvement Office (Qio) Has Developed A Capa Plan Template To Assist Study Teams With This Activity.
The first step in developing a capa plan is to identify. Web corrective action and preventive action (capa) plan template. Corrective and preventive actions (capa) plans. Web corrective and preventive actions (capa) inspectional objectives.
Web Clinical Capa Process, Managing Clinical Quality Using Corrective And Preventative Actions Is Not New To Clinical.
Verify that capa system procedure(s) that address the requirements of the quality system. Web capa is written to identify a discrepancy/problem in the conduct of a clinical research study, note the root cause of the identified problem, identify the corrective action to. A form has been created to facilitate the capa (corrective action /. Discuss all research procedures involved in the human research for each subject population participating in the study.
Web Corrective And Preventive Actions (Capa) Plans.
Web capas can be used for audit or inspection observations, compliance improvement, or risk mitigation. Web this article provides an overview of the root cause of these problems and how to ensure that corrective and preventive actions are addressing the actual problem. Guiding clinical research professionals in improving weaknesses, deficiencies, or in rectifying deviation patterns and areas of. Web five best practices.