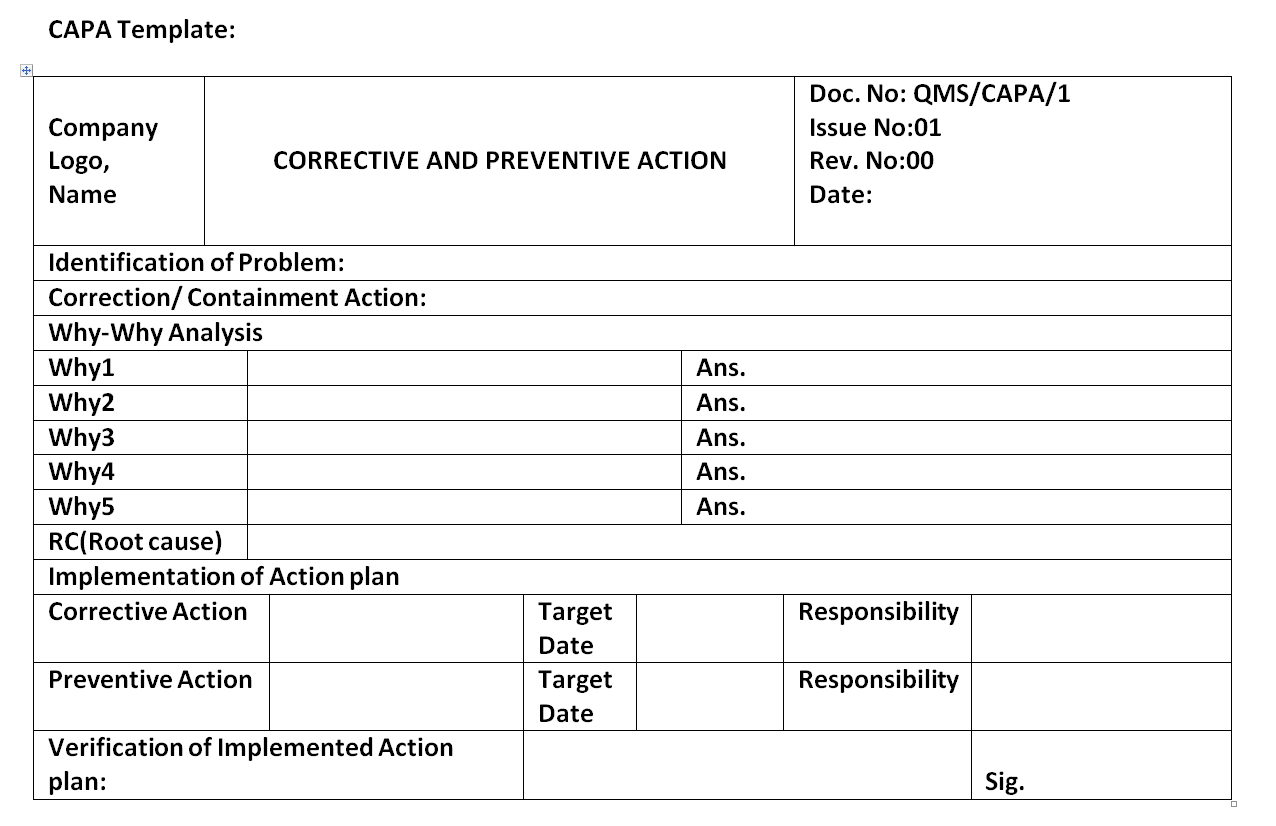
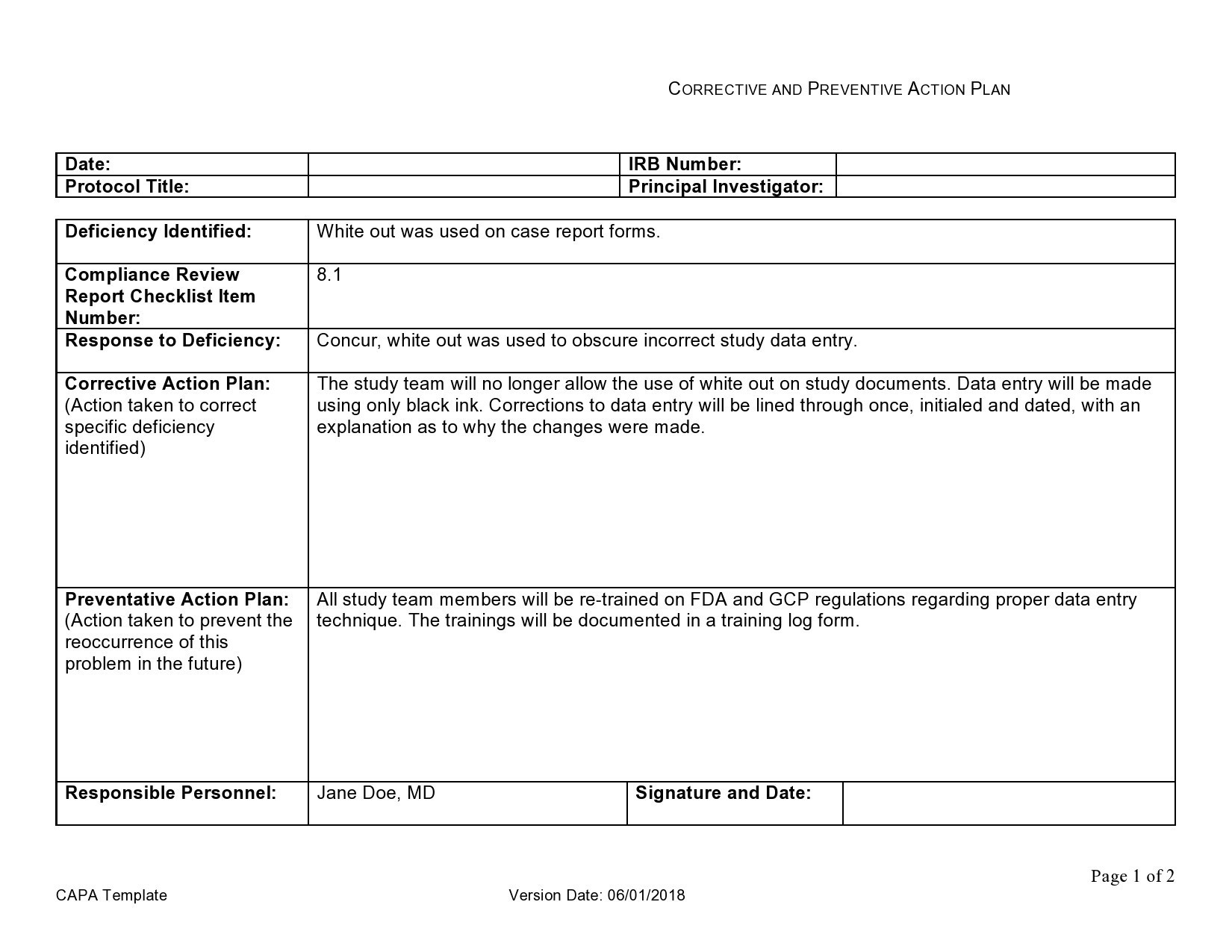
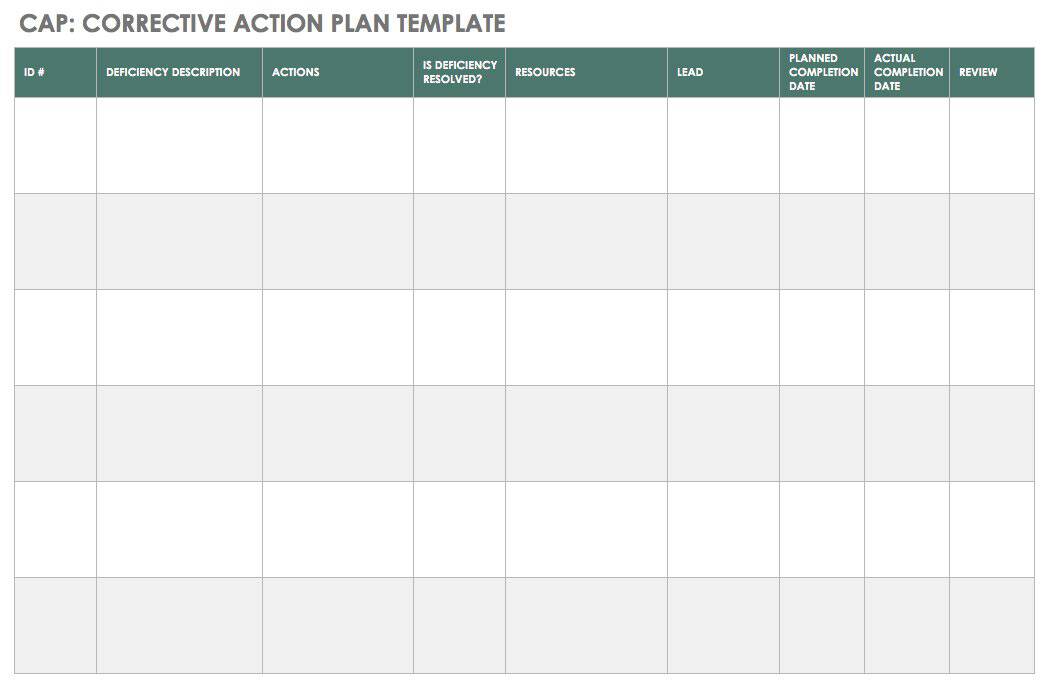
Capa Plan Template
Capa Plan Template - Edit, sign and save employee corrective action form. Web capa(corrective and preventative action) management is the most crucial component of a strong and compliant quality management system. Reporting table for unanticipated problems, adverse events, serious adverse events,. Include a process of assessing the action plan effectiveness and a process by which the plan will be amended if it is ineffective. Web corrective and preventive action (capa): Formslaw.com has been visited by 10k+ users in the past month Web the iu hrpp quality improvement office (qio) has developed a capa plan template to assist study teams with this activity. Corrective action and preventive action (capa) plan template. A capa form records the occurrence. Web it discusses capa within iso 9001 and within the regulation fda 21 cfr 820 and outlines how and what data sources serve users well in preventive action.
Pin on Example Business Form Template
Web corrective action and preventive action (capa) plan. Formslaw.com has been visited by 10k+ users in the past month Web download corrective and preventative action plan form template_2019.11.13. Web the iu hrpp quality improvement office (qio) has developed a capa plan template to assist study teams with this activity. Corrective action and preventive action (capa) plan template.
Corrective and Preventive Action Format CAPA with Example Download
A thorough capa plan must also. Formstemplates.com has been visited by 100k+ users in the past month This template is designed to. Web capa definition, the red cloak of a bullfighter, used chiefly in attracting the attention of the bull and guiding the course of its attack. Web it discusses capa within iso 9001 and within the regulation fda 21.
Sample Capa form Unique Employee Corrective Action form Action plan
A thorough capa plan must also. Web capa(corrective and preventative action) management is the most crucial component of a strong and compliant quality management system. Ad pdffiller allows users to edit, sign, fill & share all type of documents online. Reporting table for unanticipated problems, adverse events, serious adverse events,. Edit, sign and save employee corrective action form.
Capa Form Template Free Printable Form, Templates and Letter
Web the iu hrpp quality improvement office (qio) has developed a capa plan template to assist study teams with this activity. A thorough capa plan must also. Click here for a capa plan template. Reporting table for unanticipated problems, adverse events, serious adverse events,. Web corrective and preventive action (capa):
Sample Capa form Beautiful Corrective Action Report Example in 2020
Corrective and preventive actions (capa) plans. Web capa(corrective and preventative action) management is the most crucial component of a strong and compliant quality management system. The following form is used to. Web corrective and preventive action (capa): Guiding clinical research professionals in improving weaknesses, deficiencies, or in rectifying.
Pin on Example Business Form Template
Corrective action and preventive action (capa) plan template. Corrective and preventive actions (capa) plans. Ad pdffiller allows users to edit, sign, fill & share all type of documents online. Include a process of assessing the action plan effectiveness and a process by which the plan will be amended if it is ineffective. A capa form records the occurrence.
Corrective and preventive action plan CAPA report form
Corrective and preventive actions (capa) plans. Formstemplates.com has been visited by 100k+ users in the past month Web narrative medical device tracking inspectional objectives decision flow chart narrative corrective and preventive actions (capa) inspectional objectives verify. Web download corrective and preventative action plan form template_2019.11.13. Web capa definition, the red cloak of a bullfighter, used chiefly in attracting the attention.
The Beginner’s Guide to CAPA Smartsheet
Ad pdffiller allows users to edit, sign, fill & share all type of documents online. Web corrective and preventative action (capa) plan. Corrective and preventive actions (capa) plans. Formstemplates.com has been visited by 100k+ users in the past month Web download corrective and preventative action plan form template_2019.11.13.
CAPA form Corrective action and preventive action
Web it discusses capa within iso 9001 and within the regulation fda 21 cfr 820 and outlines how and what data sources serve users well in preventive action. Guiding clinical research professionals in improving weaknesses, deficiencies, or in rectifying. Web narrative medical device tracking inspectional objectives decision flow chart narrative corrective and preventive actions (capa) inspectional objectives verify. Web corrective.
Free Corrective Action Plan Template Awesome 8 Corrective Action Report
Include a process of assessing the action plan effectiveness and a process by which the plan will be amended if it is ineffective. Formslaw.com has been visited by 10k+ users in the past month Corrective and preventive actions (capa) plans. Ad pdffiller allows users to edit, sign, fill & share all type of documents online. This template is designed to.
Formstemplates.com has been visited by 100k+ users in the past month Web the following individual, designated by the pi, is responsible for documenting the problem, root cause, and capa plan, updating/revising the plan as applicable, tracking the. This template is designed to. Reporting table for unanticipated problems, adverse events, serious adverse events,. A capa form records the occurrence. Formslaw.com has been visited by 10k+ users in the past month Web download corrective and preventative action plan form template_2019.11.13. Guiding clinical research professionals in improving weaknesses, deficiencies, or in rectifying. Web corrective and preventive action (capa): Web capa definition, the red cloak of a bullfighter, used chiefly in attracting the attention of the bull and guiding the course of its attack. Identifying the root cause of. Web capa(corrective and preventative action) management is the most crucial component of a strong and compliant quality management system. Web corrective and preventative action (capa) plan. Web it discusses capa within iso 9001 and within the regulation fda 21 cfr 820 and outlines how and what data sources serve users well in preventive action. Ad pdffiller allows users to edit, sign, fill & share all type of documents online. Web narrative medical device tracking inspectional objectives decision flow chart narrative corrective and preventive actions (capa) inspectional objectives verify. Click here for a capa plan template. A thorough capa plan must also. Web the iu hrpp quality improvement office (qio) has developed a capa plan template to assist study teams with this activity. The following form is used to.
Ad Pdffiller Allows Users To Edit, Sign, Fill & Share All Type Of Documents Online.
Web the following individual, designated by the pi, is responsible for documenting the problem, root cause, and capa plan, updating/revising the plan as applicable, tracking the. The following form is used to. Corrective action and preventive action (capa) plan template. Web the iu hrpp quality improvement office (qio) has developed a capa plan template to assist study teams with this activity.
A Capa Form Records The Occurrence.
Edit, sign and save employee corrective action form. This template is designed to. Web corrective action and preventive action (capa) plan. Reporting table for unanticipated problems, adverse events, serious adverse events,.
Web Capa Definition, The Red Cloak Of A Bullfighter, Used Chiefly In Attracting The Attention Of The Bull And Guiding The Course Of Its Attack.
Include a process of assessing the action plan effectiveness and a process by which the plan will be amended if it is ineffective. Guiding clinical research professionals in improving weaknesses, deficiencies, or in rectifying. Web it discusses capa within iso 9001 and within the regulation fda 21 cfr 820 and outlines how and what data sources serve users well in preventive action. Identifying the root cause of.
Click Here For A Capa Plan Template.
Corrective and preventive actions (capa) plans. A thorough capa plan must also. Web download corrective and preventative action plan form template_2019.11.13. Web capa(corrective and preventative action) management is the most crucial component of a strong and compliant quality management system.